Hoelz Lab: Publications

Figure from the paper:
Abstract:
The nuclear pore complex (NPC) regulates transport of macromolecules into and out of the nucleus. A study now shows that mechanical force applied on the nucleus affects the transport rates across the NPC’s diffusion barrier, modulating the nuclear localization of certain cargos.
California Institute of Technology
Division of Chemistry & Chemical Engineering
1200 E. California Blvd.
Pasadena, CA 91125-7200
© Copyright Hoelz Laboratory

Forced entry into the nucleus
Petrovic, S., Hoelz, A.
(2022). Nature Cell Biology, https://doi.org/10.1038/s41556-022-00939-3


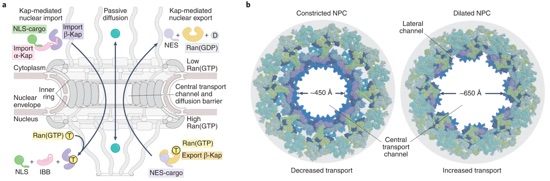
Fig. 1 | Mechanical tuning of nucleocytoplasmic transport. a, Active karyopherin (Kap)-mediated and passive transport pathways through the central transport channel of the nuclear pore complex (NPC). In the classical import pathway, the cargo’s nuclear localization signal (NLS) binds to an adaptor α-Kap (importin-α), displacing its autoinhibitory importin-β-binding (IBB) α-helix, which binds to an import β-Kap (importin-β); the ternary import complex shuttles across the diffusion barrier and disassembles upon arrival in the nucleus by Ran(GTP) binding. In the export pathway, nuclear export signal (NES)-presenting cargo forms a ternary complex with Ran(GTP) and an export β-Kap (exportin) that shuttles across the diffusion barrier; in the cytoplasm, Ran’s GTPase activity is stimulated to disassemble the complex. b, Cryo-ET reconstructions of the constricted and dilated human NPCs interpreted by docking crystal and single particle cryo-EM structures of its nucleoporin protein subunits[48] (Protein Data Bank (PDB) accessions 7TBJ, 7TBK, 7TBL, and 7TBM).