Hoelz Lab: Publications
Figure 1. Acl4 is a Chaperone of Nascent RpL4
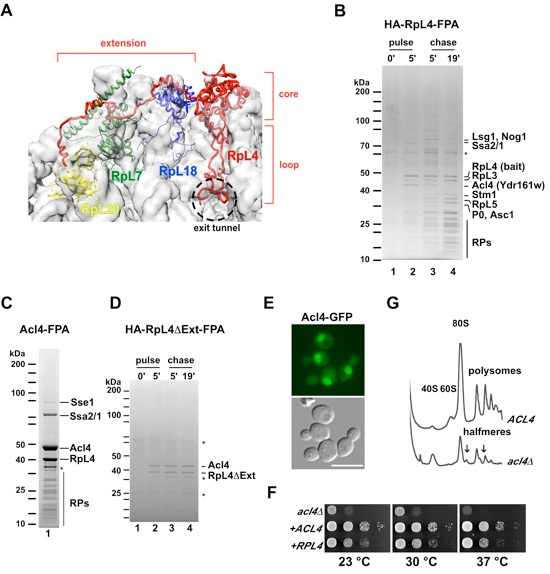
(A) Structure of RpL4 as observed in the S. cerevisiae 80S ribosome (PDB 3U5E, 3U5D) (Ben-Shem et al., 2011). Surface of 25S and 5S rRNA are shown in grey. RpL4, RpL18, RpL7 and RpL20 are shown in red, blue, green and yellow, respectively. Core, loop and C-terminal extension of RpL4 are indicated. (B) Epitope pulse-chase analysis of RpL4 in yeast cells. HA-RpL4-Flag-ProtA was pulsed for 0 (lane 1) or 5 minutes (lane 2), and chased for 5 (lane 3) and 19 minutes (lane 4). Newly synthesized RpL4 was tandem affinity-purified and analyzed by SDS-PAGE and Coomassie staining. Indicated proteins were identified by mass-spectrometry. Asterisk indicates keratin contaminant. (C) Tandem affinity-purification (Flag-ProtA) of chromosomal tagged Acl4 from yeast cells. Eluates were analyzed by SDS-PAGE, Coomassie staining and mass-spectrometry. Asterisk indicates RpL4 breakdown. (D) Epitope pulse-chase analysis of RpL4DΔExt. HA-RpL4ΔExt-Flag-ProtA was pulsed for 0 (lane 1) or 5 minutes (lane 2), and chased for 5 (lane 3) and 19 minutes (lane 4). Asterisks indicate keratin and IgG contaminants. (E) Acl4 localization in yeast cells. Acl4 was chromosomal tagged with GFP and the localization was assessed by fluorescence microscopy. Scale bar is 5 μm. (F) Growth analysis of Δacl4 strain. The impaired growth of Δacl4 was partially rescued by expression of an additional copy of RPL4 from a centromeric plasmid. (G) Polysome profiles of ACL4 and Δacl4 cells. Deletion of ACL4 impairs the synthesis of the 60S ribosomal subunit. Free 80S, 60S, 40S and polysomes are indicated. Arrows point to the observed halfmer polysomes. See also Figure S1.

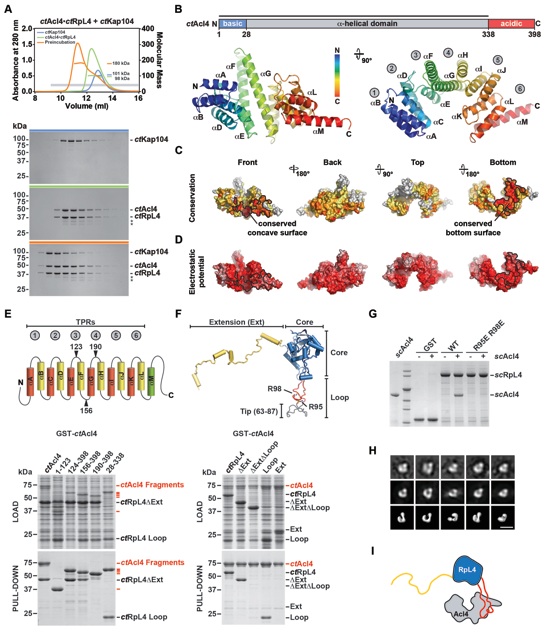
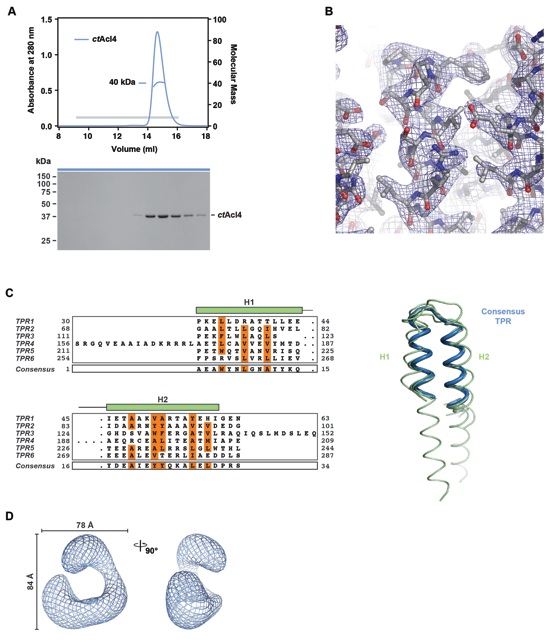
Figure 2. Biochemical and Structural Analysis of the Acl4-RpL4 Interaction (A) Size-exclusion chromatography coupled to multiangle light scattering (SEC-MALS) analysis of the ctAcl4•ctRpL4•ctKap104 trimeric nuclear import complex. The absorbance at 280 nm is plotted against the elution volume of a Superdex 200 10/300 GL size exclusion column and overlaid with the molecular mass of the different proteins. Fractions that were resolved on a SDS-PAGE gel and visualized by Coomassie staining are indicated by a gray bar. Degradation products are labeled with asterisks. (B) Domain representation and crystal structure of ctAcl4. Blue, basic N-terminal region; grey, α-helical region; red, acidic C-terminal region. The crystallized fragment is indicated with a black bar. The ctAcl4 crystal structure is shown in cartoon representation in two different orientations. (C) Surface representation of ctAcl4 in four different orientations colored according to a multi-species sequence alignment (Figure S3A). Sequence conservation is shaded from white (< 40 % similarity) to yellow (40 % similarity) to red (100 % identity). (D) Surface representation colored according to electrostatic potential from -10 kBT/e (red) to +10 kBT/e (blue). (E) GST pull-down of ctAcl4 variants. Red, GST-ctAcl4 variants (bait); black, ctRpL4 variants. Loaded (top, soluble lysate fraction) and pulled-down (bottom) fractions were analyzed by SDS-PAGE and Coomassie staining. ctAcl4 fragment boundaries are shown above each lane and are depicted in a cartoon. (F) GST pull-down of ctRpL4 variants. Red, GST-ctAcl4 (bait); black, ctRpL4 variants. ctRpL4 fragment boundaries are shown above each lane and indicated in the scRpL4 structure extracted from the S. cerevisiae ribosome (PDB 1VXY) (Svidritskiy et al., 2014). (G) His-scAcl4, GST-scRpL4 and GST-scRpL4 R95E/R98E were purified from E. coli. GST, GST-scRpL4 and GST-scRpL4 R95E/R98E beads were incubated with excess of imidazole eluted His-scAcl4. Beads were boiled and eluates were resolved on a SDS-PAGE gel and visualized by Coomassie Brilliant Blue staining. (H) Negative stain electron microscopic analysis of recombinant purified ctAcl4•ctRpL4 complex. Two-dimensional class average of ctAcl4•ctRpL4 complex (top row) determined by multivariate statistical analysis matching with the projections of the final 3D model (middle row) and surface representations of equivalent orientations (bottom row). (I) Model of the Acl4•RpL4 complex. See also Figure S2 and S3.
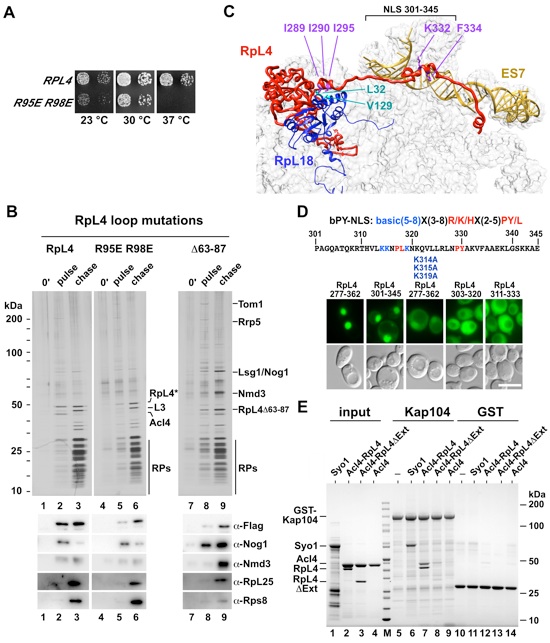
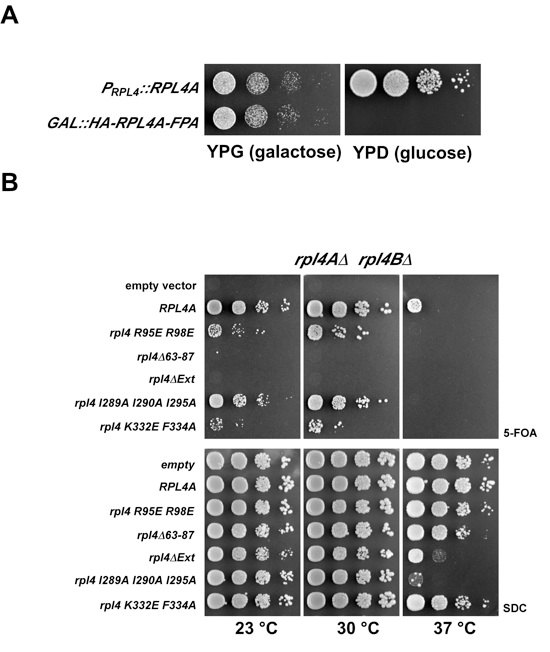
Figure 3. (A) rpl4AΔ/rpl4BΔ knockout strain was transformed with an empty vector or vectors containing RPL4A and RPL4A R95E/R98E. The RPL4A wild-type copy on the URA-plasmid was shuffled out on FOA. The respective clones were spotted on YPD plates and incubated at 23 °C, 30 °C and 37 °C for 2 days. (B) Epitope pulse-chase analysis of RpL4, RpL4 R95E/R98E and RpL4 Δ63-87. Wild-type (lanes 1-3) or mutants (lanes 4-9) of RpL4 were pulsed for 0 minutes (lanes 1, 4, 7) or 5 minutes (lanes 2, 5, 8) as a GAL::tcapt-HA-RpL4-Flag-ProtA version and subsequently chased for 19 minutes (lanes 3, 6, 9). HA-RpL4-Flag-ProtA was affinity-purified and resolved on a SDS-PAGE gel and visualized by silver staining or western blot (lower panel). Indicated proteins were identified by mass-spectrometry. Acl4 was not found in the purification of RpL4 R95E/R98E and RpL4Δ63-87 (lanes 5, 8). The original blot and silver SDS-PAGE gel were sliced and put together from one gel/blot image (original blot and SDS-PAGE gel, see Figure S5). Accordingly, wild-type RpL4 (lanes 1-3) in Figures 3B and 4B are derived from the same gel. (C) Part of the 60S subunit with RpL4, RpL18 and the extended sequence of 25S RNA (ES7) are depicted. The RpL4 residues I289, I290 and I295 (violet) contacting RpL18 residues L32 and V129 (cyan), and RpL4 residues K332 and F334 (violet) contacting ES7 RNA (goldenrod) are shown. (D) The C-terminal extension (residues 277-362) of RpL4 targets the attached 3xyEGFP reporter to the nucleus. A 44-residue region of the C-terminal RpL4 extension (residues 301-345), containing two potential PY-NLS sequences (consensus is indicated above the amino acid sequence), is sufficient for efficient nuclear import. Mutation of three lysine residues (K314A, K315A, K319A) in the extended NLS (residues 277-362) or shorter constructs, ranging from residues 303-320 or 311-333, were also tested for NLS activity by monitoring nuclear accumulation of GFP. Scale bar is 5 μm. (E) The scKap104 interaction with scRpL4 is dependent on its C-terminal extension. GST-scKap104, His6-scAcl4•scRpL4, His6-scAcl4•scRpL4ΔExt, His6-scAcl4 and His6-scSyo1 (positive control for scKap104 binding) (Kressler et al., 2012) were expressed in E. coli and affinity-purified (lanes 1-5, input). GST-scKap104 (lanes 5-9) and GST (lanes 10-14) were immobilized on GSH-beads and incubated with excess of purified His6-scAcl4•scRpL4, His6-scAcl4•scRpL4ΔExt, His6-scAcl4 and His6-scSyo1. Samples were analyzed by SDS-PAGE and Coomassie staining. See also Figure S4 and S5.
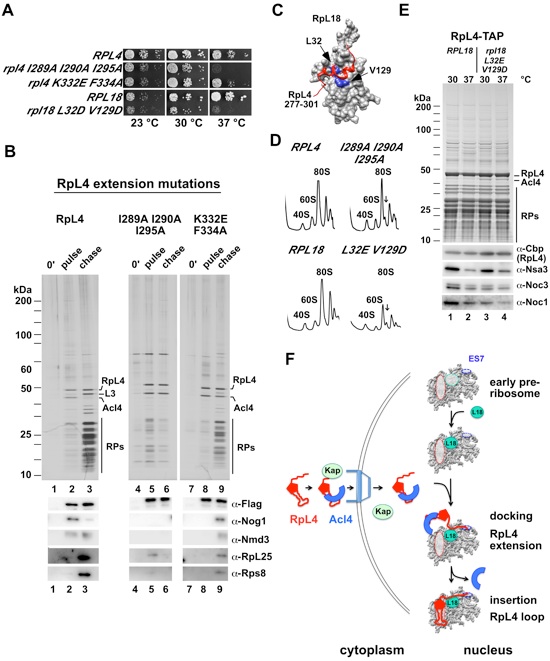
Figure 4. The C-Terminal Extension of RpL4 Coordinates the Incorporation of RpL4 into the Pre-Ribosome (A) Growth analysis of RPL4 (wt), RPL4-RPL18 contact and RPL4-ES7 contact mutants. (B) Epitope pulse-chase analysis of RpL4 (lanes 1-3) and RpL4-RpL18 contact mutant (I289A, I290A, and I295A) (lane 4-6) and RpL4-ES7 contact mutant (K332E and F334A) (lanes 7-9). RpL4 and RpL4 mutants were pulsed for 0 minutes (lanes 1, 4, 7) or 5 minutes (lanes 2, 5, 8) as a GAL::tcapt-HA-RpL4-Flag-ProtA version and subsequently chased for 19 minutes (lanes 3, 6, 9), tandem affinity-purified (TAP) and separated by SDS-PAGE and analyzed by silver staining and western blot. Blot and silver gel were sliced and put together from one gel/blot image (original blot and gel, see Figure S5). Accordingly, wild-type RpL4 (lanes 1-3) in Figures 3B and 4B are derived from the same gel. (C) Close up view of the RpL4-RpL18 interaction, as observed in the S. cerevisiae 80S ribosome (PDB 3U5E) (Ben-Shem et al., 2011). Surface depiction of RpL18 (grey) and ribbon illustration of the RpL18 contact region of the C-terminal RpL4 extension (residues 277-301, red). Mutated RpL18 residues are indicated in blue (PDB 3U5E, 3U5D) (Ben-Shem et al., 2011). (D) Ribosome profiles of wild-type and rpl4 I289A I290A I295A or rpl18 L32E V129D (RpL4-RpL18 contact mutant) strains analyzed by sucrose gradient centrifugation of cell homogenate. (E) Tandem affinity-purification (TAP) of chromosomal tagged RpL4 in RPL18 (lanes 1 and 2) and rpl18 L32E V129D (lanes 3 and 4) strains. Purified samples were separated by SDS-PAGE and analyzed by Coomassie staining and western blot (lower panel). Cells (lanes 2 and 4) were shifted to 37 °C for 2.5 hours. The band labeled as Acl4 was identified by mass spectrometry. (F) Model of coordinated RpL4 assembly into the maturing ribosome. Details as discussed in the text. See also Figure S4 and S5.
Figure SI 1. Growth analysis of RPL4 wild-type and rpl4 mutants in a rpl4AΔ rpl4BΔ shuffle strain (related to Figure 1). (A) Growth complementation of HA-RPL4-FPA. pGAL::HA-RPL4A-FPA or pPRPL4-RPL4A were transformed into rpl4AΔ rpl4BΔ shuffle strain and plated on SC-galactose plates. Transformants were transferred on 5-FOA plates and after 3 days spotted on YP-galactose and YP-glucose plates. It was incubated for 3 days at 30 °C. (B) Growth analysis of rpl4 mutants. The indicated wild-type RPL4A and rpl4A mutants were cloned into centromeric plasmids under the endogenous RPL4 promoter. Plasmids were transformed into rpl4AΔ rpl4BΔ shuffle strain. Transformants were spotted on 5-FOA and SC plates for 3 days at 23 °C, 30 °C and 37 °C.
PDB coordinates (link to PDB site) - Acl4 1-338, Acl4 28-338
PDBCoordinates (*.pdb) -Acl4 1-338, Acl4 28-338
Structure Factors (.txt) - Acl4 1-338, Acl4 28-338
Figures from the paper:
Coordinates:
Abstract:
Eukaryotic ribosome biogenesis requires nuclear import and hierarchical incorporation of 80 ribosomal proteins (RPs) into the ribosomal RNA core. In contrast to prokaryotes, many eukaryotic RPs possess long extensions that interdigitate in the mature ribosome. RpL4 is a prime example, with an 80-residue-long surface extension of unknown function. Here, we identify assembly chaperone Acl4 that initially binds the universally conserved internal loop of newly synthesized RpL4 via its superhelical TPR domain, thereby restricting RpL4 loop insertion at its cognate nascent rRNA site. RpL4 release from Acl4 is orchestrated with pre-ribosome assembly, during which the eukaryote-specific RpL4 extension makes several distinct interactions with the 60S surface, including a co-evolved site on neighboring RpL18. Consequently, mutational inactivation of this contact site, on either RpL4 or RpL18, impairs RpL4-Acl4 disassembly and RpL4 pre-ribosome incorporation. We propose that hierarchical ribosome assembly can be achieved by eukaryotic RP extensions and dedicated assembly chaperones.
California Institute of Technology
Division of Chemistry & Chemical Engineering
1200 E. California Blvd.
Pasadena, CA 91125-7200
© Copyright Hoelz Laboratory

Coordinated Ribosomal L4 Protein Assembly into the Pre-Ribosome Is Regulated by Its Eukaryote-Specific Extension
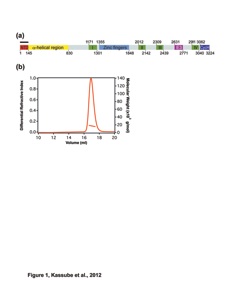
Figure S2. Further analyses of the ctAcl4 crystal structure (related to Figure 2). (A) Size-exclusion chromatography coupled to multiangle light scattering (SEC-MALS) analysis of the ctAcl428-338 fragment. The absorption at 280 nm is plotted against the elution volume from a Superdex 200 10/300 GL gel filtration column and overlaid with the observed molecular mass. The grey bar indicates fractions that were resolved on a SDS-PAGE gel and visualized by Coomassie staining. (B) Representative section of final 2|Fo|-|Fc| electron density map contoured at 1.0 σ. (C) Structural superposition of the six ctAcl4 TPRs and a canonical TPR (PDB 2AVP) (Kajander et al., 2007). Structure-guided sequence alignment of ctAcl4 TPRs and the canonical TPR. Consensus hydrophobic sequences are highlighted in orange. (D) Selected views of the volume representation of the ctAcl4•ctRpL4 complex.
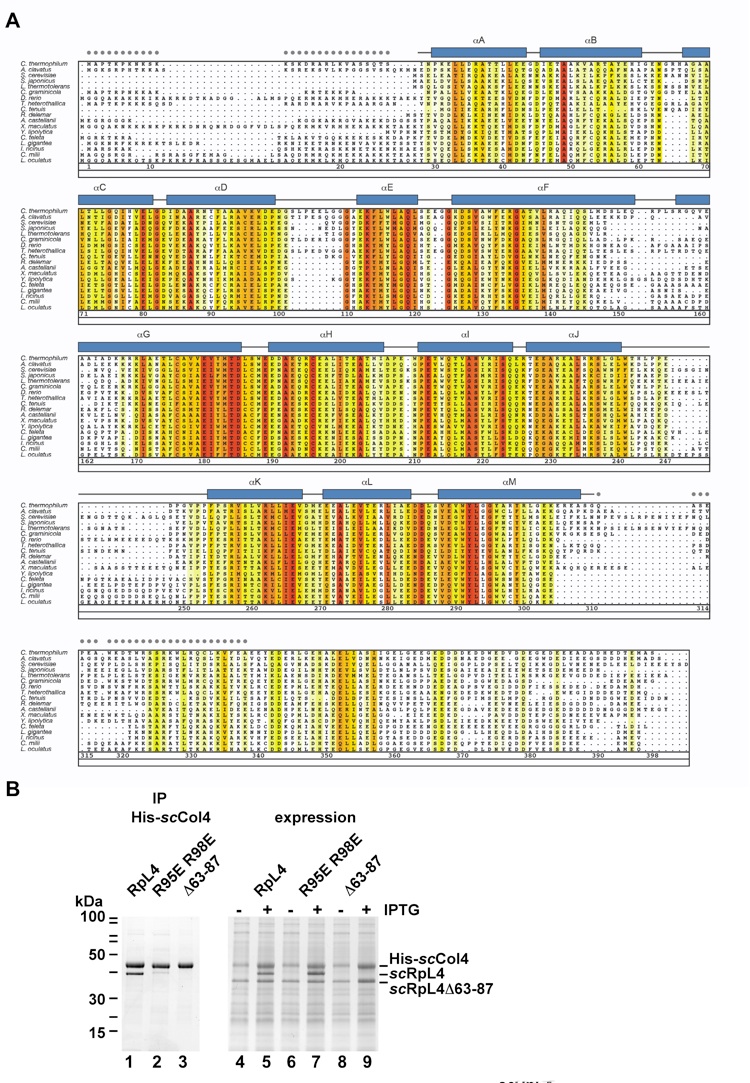
Figure S3. Figure S3. Acl4 homologs are found up to vertebrates and RpL4 loop mutants are defective in Acl4 binding (related to Figures 2 and 3). (A) Acl4 protein sequences from 18 species were aligned and colored according to a Blosum62 matrix with white (< 40 % similarity), yellow (40 % similarity) to dark red (100 % identity). The residue numbering is according to the ctAcl4 sequence. The α-helical regions are represented as blue rectangles and are numbered from αA to αM. Gray dots indicate residues of the crystallized construct that were not observed in the electron density map and are presumed to be disordered. (B) RpL4 loop mutants are impaired in Acl4 binding. Co-expression of His6-scAcl4•scRpL4, His6-scAcl4•scRpL4 R95E/R98E loop mutant and His6-scAcl4•scRpL4Δ63-87 in E. coli. Right panel shows whole cell lysates before and after IPTG induction (lanes 4-9). The imidazole eluates (lanes 1-3) of purified His6-scAcl4•scRpL4, His6-scAcl4•scRpL4 R95E/R98E loop mutant and His6-scAcl4•scRpL4Δ63-87 were analyzed by SDS-PAGE and Coomassie staining.
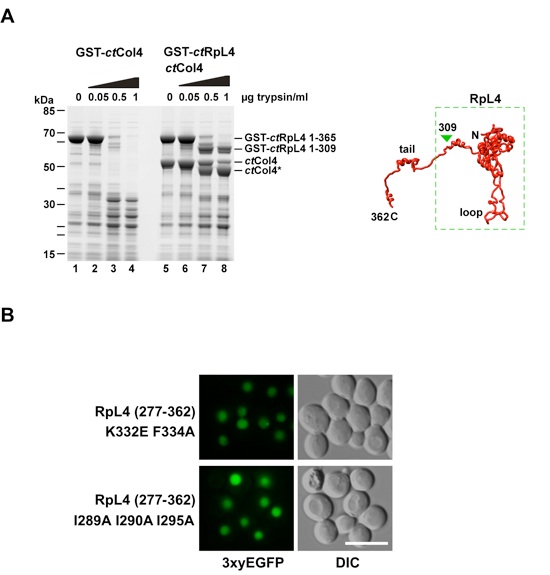
Figure S4. RpL4-Acl4 interaction stabilizes the RpL4 core/loop but not the extension from tryptic digest, and mutations in the RpL4 extension impairing ribosome interaction still allow nuclear import (related to Figures 2 and 4). (A) Tryptic digest of ctRpL4 and ctAcl4•ctRpL4. GST-ctRpL4 and His6-ctAcl4 were purified from E. coli. GST-ctRpL4 was immobilized on GSH-beads and incubated with excess of His6-ctAcl4 (lanes 5-8). After washing, beads were incubated with increasing amounts of trypsin for 30 minutes at 37 °C. Beads were boiled with sample buffer and analyzed by SDS-PAGE and Coomassie staining. Tryptic fragments were analyzed by Orbitrap mass-spectrometry. Right panel illustrates the RpL4 (yeast) structure with the stable fragment highlighted by the dashed box (Ben-Shem et al., 2011). (B) The RpL4 extension mutants, RpL4277-362 K332E/F334A and RpL4277-362 I289A/I290A/I295A, target the 3xyEGFP reporter efficiently to the nucleus. Scale bar is 5 μm.
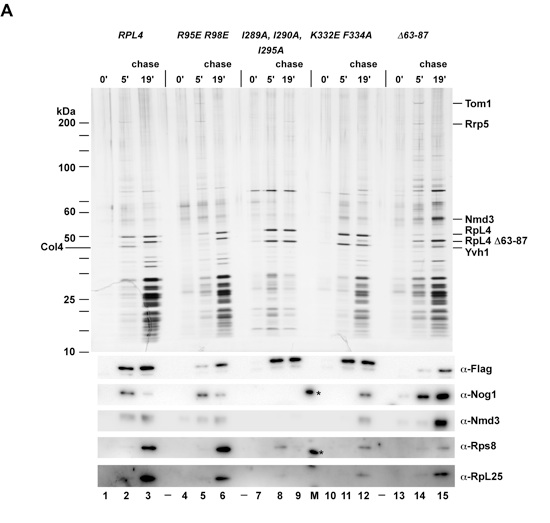
Figure S5. Original silver stained polyacrylamide gel (uncut) and Western blots (related to Figures 3 and 4). Epitope pulse-chase analysis of RpL4 (lanes 1-3), RpL4 R95E/R98E (lanes 4-6) and RpL4 I289A/I290A/I295A (lanes 7-9), RpL4 K332E/F334A (lanes 10-12), RpL4 Δ63-87 (lanes 13-15). RpL4 and RpL4 mutants were pulsed for 0 minutes (lanes 1, 4, 7, 10, 13) or 5 minutes (lanes 2, 5, 8, 11, 14) based on GAL::tcapt-HA-RpL4-Flag-ProtA constructs and subsequently chased for 19 minutes (lanes 3, 6, 9, 12, 15), followed by tandem affinity-purification and SDS-PAGE. Shown is the initial SDS-PAGE gel, which was stained with silver (upper panel), and derived Western blots using the indicated antibodies (lower panels). Gel and blots were cut and arranged for better illustration of the data (see Figure 3B and 4B). Antibody cross-reaction with standard protein bands (M) is indicated by asterisks.



Stelter, P.,# Huber, F.M.,# Kunze, R., Flemming, D., Hoelz, A.*, Hurt, E.*
(2015). Mol. Cell, 58, 854-862