Hoelz Lab: Publications
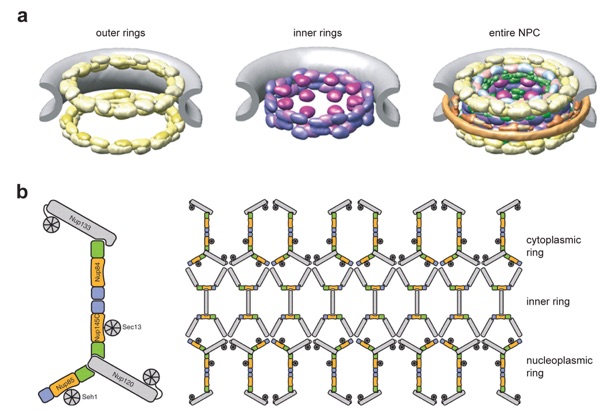
Figure 1. Overall structure of the nuclear pore complex (NPC). (a) Cryo-electron tomographic reconstruction of the Dictyostelium discoideum NPC [Electron Microscopy Data Bank (EMDB) code 1097, Reference 81]. The cytoplasmic filaments, the symmetric core, and the nuclear basket are colored in cyan, orange, and purple, respectively. (b) A schematic model of the NPC. The four concentric cylinders are composed of integral pore membrane proteins (POMs), coat nucleoporins, adaptor nucleoporins, and channel nucleoporins. Natively unfolded phenylalanine-glycine (FG) repeats of a number of nucleoporins make up the transport barrier in the central channel and are indicated by a transparent plug.

Hoelz, A.*,**, Debler, E.W., Blobel, G. (2011). Annu. Rev. Biochem. 80, 613-643.
* Corresponding author; ** invited author
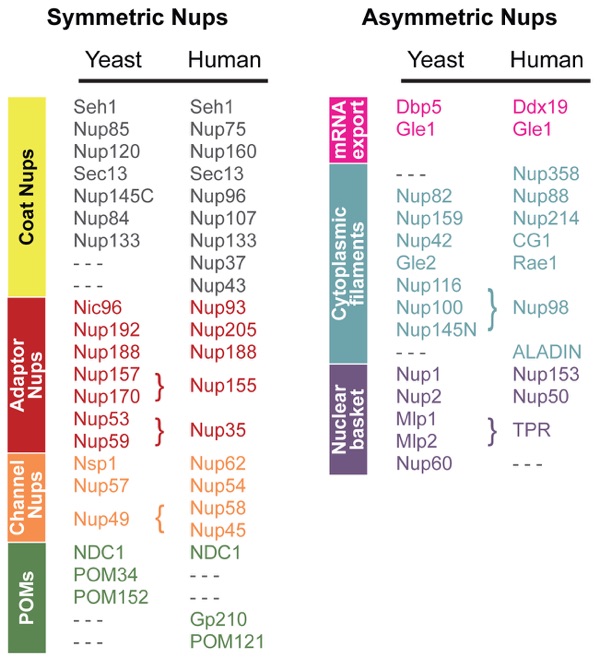
Figure 2. Molecular composition of the yeast and human nuclear pore complexes (NPCs). Symmetric nucleoporins form the core region and are equally distributed on the cytoplasmic and nucleoplasmic halves of the NPC. Asymmetric nucleoporins form the nuclear basket and the cytoplasmic filaments that serve as docking sites for transport factors and include associated mRNA export factors. The nucleoporin classification is as described in Figure 1b. POM, an integral membrane protein.
Figures from the paper:
Abstract:
In eukaryotic cells, the spatial segregation of replication and transcription in the nucleus and translation in the cytoplasm imposes the requirement of transporting thousands of macromolecules between these two compartments. Nuclear pore complexes (NPCs) are the sole gateways that facilitate this macromolecular exchange across the nuclear envelope with the help of soluble transport receptors. Whereas the mobile transport machinery is reasonably well understood at the atomic level, a commensurate structural characterization of the NPC has only begun in the past few years. Here, we describe the recent progress toward the elucidation of the atomic structure of the NPC, highlight emerging concepts of its underlying architecture, and discuss key outstanding questions and challenges. The applied structure determination as well as the described design principles of the NPC may serve as paradigms for other macromolecular assemblies.
Structure of the Nuclear Pore Complex



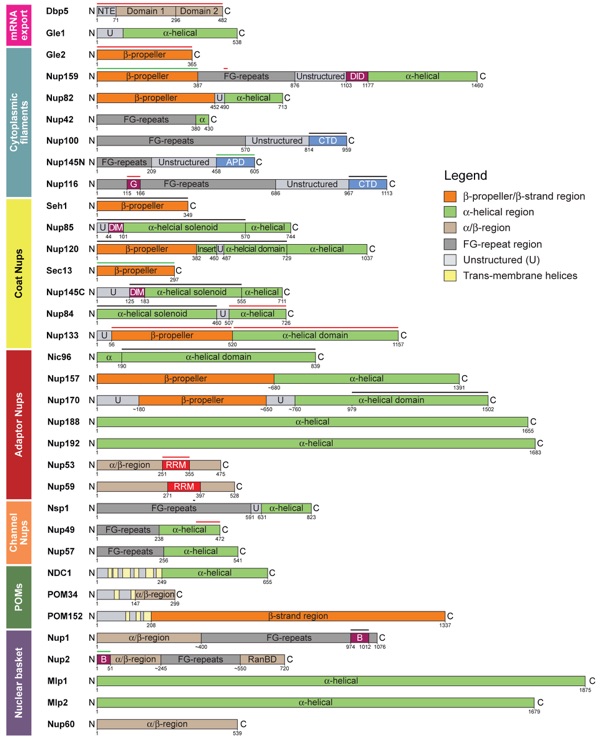
Figure 3. Domain architecture of yeast nucleoporins. Domain borders are indicated by residue numbers. NTE, N-terminal extension; U, unstructured; FG repeat, phenylalanine-glycine (FG) repeat; DID, dynein light-chain interacting domain; CTD, C-terminal domain; G, Gle2-binding sequence (GLEBS); APD, autoproteolytic domain; DIM, domain invasion motif; RRM, RNA-recognition motif; B, karyopherin-binding domain; RanBD, Ran-binding domain; POM, an integral membrane protein. The bars above the domain organizations mark fragments whose crystal structures are experimentally determined (black, yeast; red, mammalian; green, yeast and mammalian). Notably, the nucleoporins Nup1 and Nup2 do not follow the common nomenclature because the numbers 1 and 2 do not refer to their molecular weight.
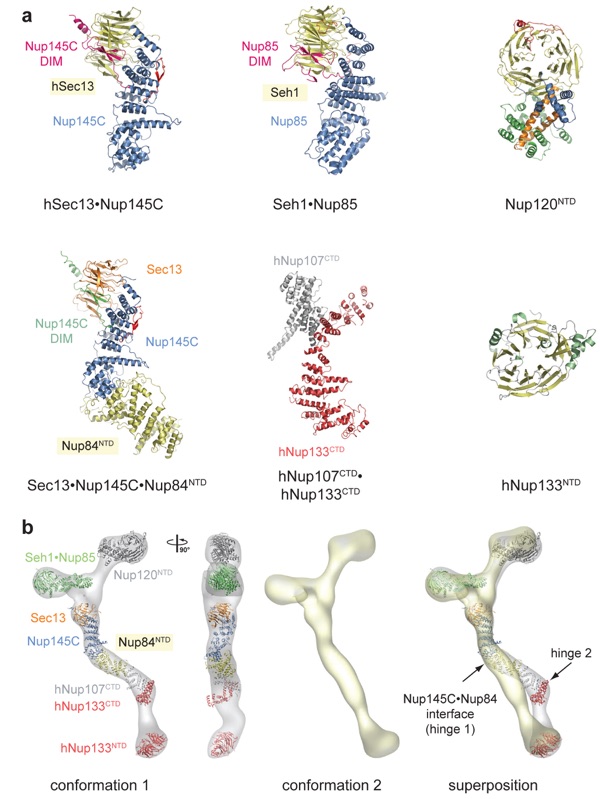
Figure 4. Structural characterization of the heptameric Nup84 complex. The prefix h refers to human; protein names without a prefix refer to yeast. (a) Crystal structures of coat nucleoporins and complexes thereof: hSec13·Nup145C [Protein Data Bank (PDB) codes 3BG1, 3BG0], Seh1·Nup85 (3F3F, 3F3G, 3F3P, 3EWE), Nup120NTD (N-terminal domain) (3F7F, 3H7N, 3HXR), Sec13·Nup145C·Nup84NTD (3IKO, 3JRO), hNup107CTD·hNup133CTD (C-terminal domain) (3I4R, 3CQC, 3CQG), and hNup133NTD (1XKS). For those structures with several PDB codes, the first one refers to the displayed structure. (b) Docking of crystal structures into the electron microscopy (EM) envelope of the heptameric Nup84 complex (first panel). A 90°-rotated view is shown (second panel). The EM envelope of the second reconstructed conformation of the heptamer in which the two hinge regions are completely extended (third panel). Superposition of the two determined Nup84 conformations (fourth panel). The hinge region at the Nup145C·Nup84 interface is indicated and was used for the structural alignment.
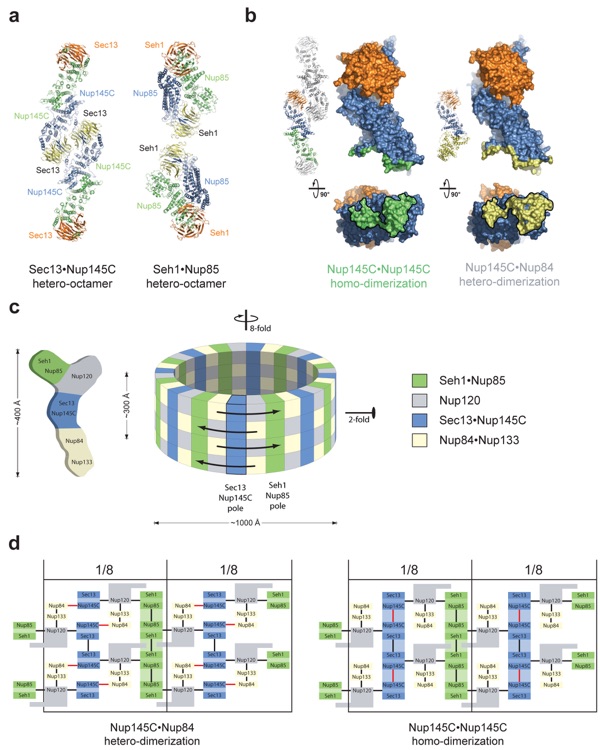
Figure 5. Model for the architecture of a “coat for the nuclear pore membrane.” (a) Structures of the hSec13·Nup145C and Seh1·Nup85 hetero-octamers. (b) Binding promiscuity of Nup145C. Nup145C·Nup145C homodimerization in the hSec13·Nup145C hetero-octamer (panel a, left) and Nup145C·Nup84 heterodimerization in the Sec13·Nup145C·Nup84NTD heterotrimer (right). (c) Schematic representation of the heptameric Nup84 complex and the approximate localization of its seven nucleoporins (left). Eight heptamers are circumferentially arranged in a head-to-tail fashion in four stacked rings, thereby forming a cylindrical scaffold (right). (d) Two alternative states of the coat for the nuclear pore membrane. Black lines indicate interactions that have not been described as promiscuous. In one state (left), Nup145C heterodimerizes with Nup84 (red lines). In another state (right), Nup145C homodimerizes (red lines) and forms a vertical hetero-octameric pole. Note that large protein rearrangements would not be necessary to convert between the two states.
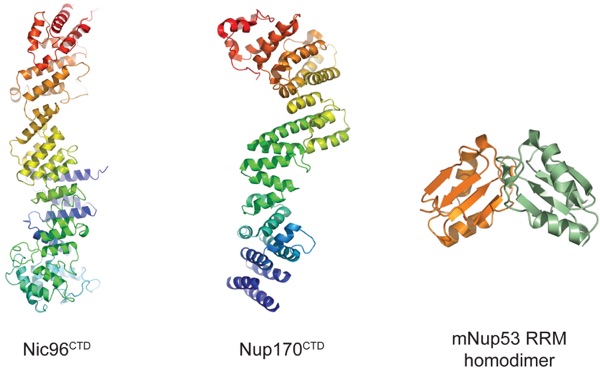
Figure 6. Crystal structures of adaptor nucleoporins: Nic96CTD (C-terminal domain) (2RFO, 2QX5), Nup170CTD (3I5P, 3I5Q), and the mNup35 RNA-recognition motif (RRM) homodimer (1WWH). The ribbon representations of Nic96CTD and Nup170CTD are shown in rainbow colors from blue to red along the polypeptide chain from the N to the C terminus. Protein Data Bank codes are in parentheses. The prefix m refers to mouse; protein names without a prefix refer to yeast.
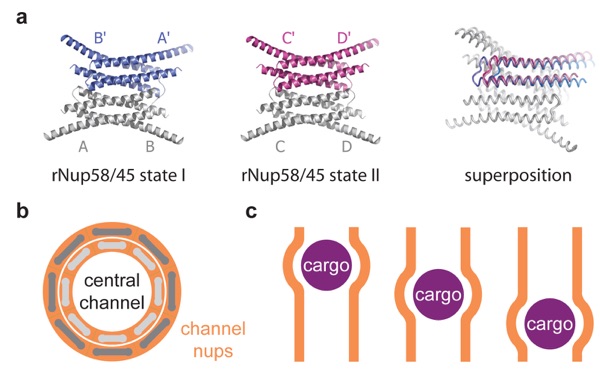
Figure 7. Crystallographic analysis of the channel nucleoporin Nup58. (a) Ribbon representations of two tetrameric rat rNup58 conformers (left and middle). Superposition of the two tetrameric rNup58 states (right) reveals a lateral shift in which two dimers are offset by up to two helical turns. Note that the crystallized fragment refers to a shared region of Nup58 and its splice variant Nup45. (b) Schematic representation of the central channel shown along the nucleocytoplasmic axis. Eight rNup58 tetrameric assemblies are circularly arranged to form a ring (light gray). Simultaneous helical sliding of the eight rNup58 tetramers would result in the overall dilation of the central channel (dark gray). (c) Localized changes in the channel diameter by α-helical sliding in response to cargo transport (purple) across the central channel (orange).
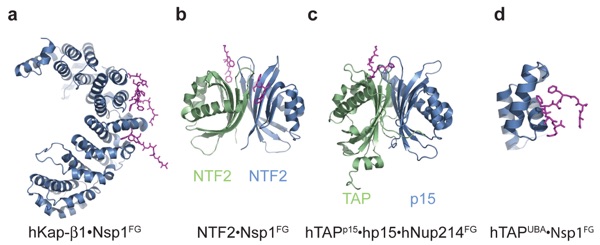
Figure 8. Crystal structures of transport factors in complex with FG repeats: (a) hKap-β1·Nsp1FG (1F59, 1O6O, 1O6P), (b) NTF2·Nsp1FG (1GYB), (c) hTAPp15·hp15·hNup214FG, and (d) hTAPUBA·Nsp1FG (1OAI). FG repeats are illustrated in purple stick representation. Protein Data Bank codes are in parentheses. The prefix h refers to human; protein names without a prefix refer to yeast.
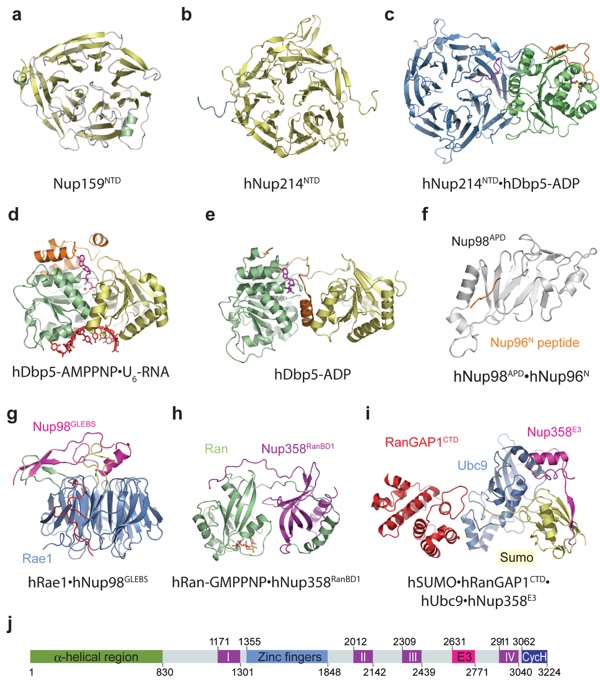
Figure 9. Crystal structures of cytoplasmic-filament nucleoporins and associated mRNA export factors: (a) Nup159NTD (1XIP), (b) hNup214NTD (2OIT), (c) hNup214NTD·hDbp5-ADP (3FMO, 3FMP, 3FHC), (d) hDbp5-AMPPNP·U6-RNA (3FHT, 3G0H), (e) hDbp5-ADP (3EWS), (f) hNup98APD·hNup96N (1KO6), (g) hRae1·hNup98GLEBS (3MMY), (h) hRan-GMPPNP·hNup358RanBD1 (1RRP), (i) hSUMO·hRanGAP1CTD·hUbc9·hNup358E3 (1Z5S), and (j) domain organization of hNup358. APD, autoproteolytic domain; CycH, cyclophilin homology domain; CTD, C-terminal domain; E3, E3 ligase domain; GLEBS, Gle2-binding sequence; I−IV, Ran-binding domains; NTD, N-terminial domain; RanBD, Ran-binding domain. Protein Data Bank codes are in parentheses. The prefix h refers to human; protein names without a prefix refer to yeast.
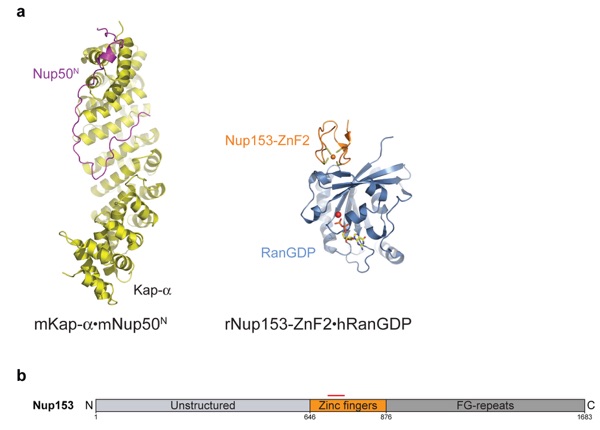
Figure 10. (a) Crystal structures of basket nucleoporins: mKap-α·mNup50N (2C1M) and rNup153ZnF2·hRanGDP (3CH5, 3GJ3–8). The Zn and Mg ions are represented as orange and red spheres, respectively. (b) Domain organization of hNup153. The red bar marks the fragment whose crystal structure is experimentally determined. Protein Data Bank codes are in parentheses. N, N-terminal domain; h, human; m, mouse; r, rat.
Figure 11. Alternative models of the architecture of the symmetric nuclear pore complex (NPC) core. (a) The “computational model” and (b) the “lattice model.” For details, see description in the text. Images are reprinted with permission from References 67 and 14, respectively.
Movie 1. Overall, the nuclear pore complex (NPC) consists of a cylindrical, symmetric core, which is asymmetrically decorated with filaments and a nuclear basket structure on the cytoplasmic and nucleoplasmic sides, respectively. Molecules smaller than ∼40 kDa (small spheres) freely diffuse through the NPC, whereas larger noncargo molecules (large spheres) are prevented from crossing the nuclear envelope. Artwork by Joseph Alexander, Erik W. Debler, and André Hoelz.
Movie 2. Active import and export of cargoes are facilitated by nuclear localization and nuclear export sequences (NLS and NES, respectively) that are recognized by transport factors, collectively termed karyopherins (Kaps). The NLS of import cargoes (blue) is recognized either directly by an import karyopherin-β (Kap-β; salmon) or via an adapter karyopherin (Kap-α; light green). RanGTP (red) binding inside the nucleus leads to dissociation of the import complex. By contrast, the assembly of a NES-cargo Kap-β export complex requires RanGTP binding (represented in blue, yellow, and red, respectively). In the cytosol, this export complex is dissociated by GTP hydrolysis, which is catalyzed by Ran GTPase-activating protein (RanGAP; dark green) or Ran-binding protein 1 (RanBP1). Artwork by Joseph Alexander, Erik W. Debler, and André Hoelz.
Movie 3. The transport of large cargoes (blue) is thought to require the dilation of the central channel of the NPC. Artwork by Joseph Alexander, Erik W. Debler, and André Hoelz.
Movie 4. Inner nuclear membrane (INM) proteins are cotranslationally integrated into the endoplasmic reticulum membrane, which is continuous with the outer nuclear membrane, and then imported to the INM. Similar to the karyopherin-mediated transport in Movie 2, the transport of INM proteins is also dependent on the Ran cycle and karyopherins that likely travel through the central channel, whereas the cargo protein is anchored in the membrane. INM proteins, Kap-α, Kap-β, and RanGTP, are illustrated in light purple, light green, brown, and red, respectively. Substantial structural changes within the NPC would be necessary to facilitate this transport event. Artwork by Joseph Alexander, Erik W. Debler, and André Hoelz.

California Institute of Technology
Division of Chemistry & Chemical Engineering
1200 E. California Blvd.
Pasadena, CA 91125-7200
© Copyright Hoelz Laboratory
