Hoelz Lab: Publications
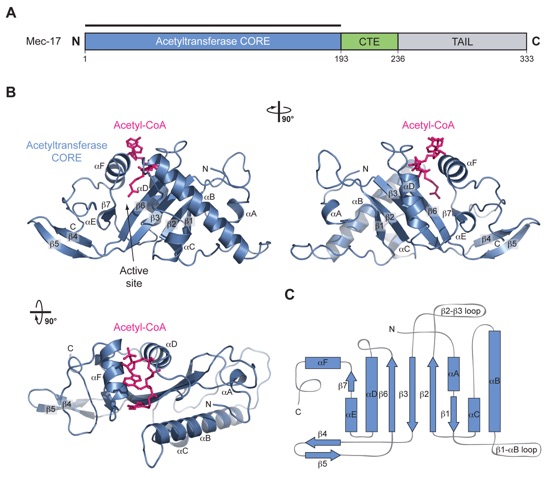
Figure 1. Structural Overview of MEC-17. (a) Domain structure. Blue, acetyltransferase domain; Green, C-terminal extension (CTE); Gray, unstructured C-terminal tail. (b) Cartoon representation of the MEC-17COREacetyl-CoA structure, shown rotated 90º to the right and bottom. (c) Schematic representation of the architecture of MEC-17CORE with secondary structure elements indicated.

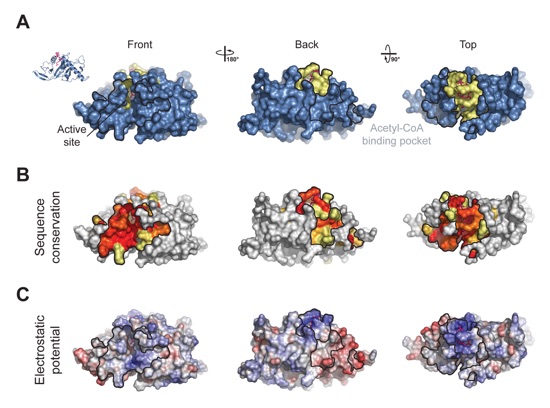
Figure 2. Surface Properties of MEC-17. (a) Surface representation colored according to identified binding regions, with the acetyl-CoA binding pocket in yellow. (b) Surface representation of MEC-17CORE colored according to a multispecies sequence alignment. The conservation at each position is mapped onto the surface and is shaded in a color gradient from white (below 60 % identity) to light yellow (60 % identity) to dark red (100 % identity). (c) Surface representation of MEC-17CORE colored according to electrostatic potential. The electrostatic potential is plotted onto the surface and colored in a gradient from red (-10 kBT/e) to blue (10 kBT/e).
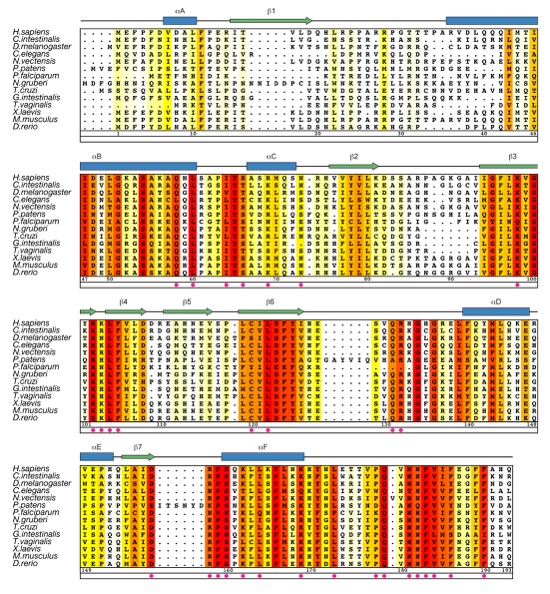
Figure 3. Multi-species Sequence Alignment of MEC-17 Homologues. The secondary structure is indicated above the sequence as blue cylinders (α helices), green arrows (β sheets), and gray lines (coil regions). The numbering below the alignment is relative to human MEC-17. Overall sequence conservation at each position is shaded in a color gradient from white (below 60 % identity) to yellow (60 % identity) to dark red (100 % identity). Magenta dots indicate mutated residues in our biochemical and in vivo analyses.
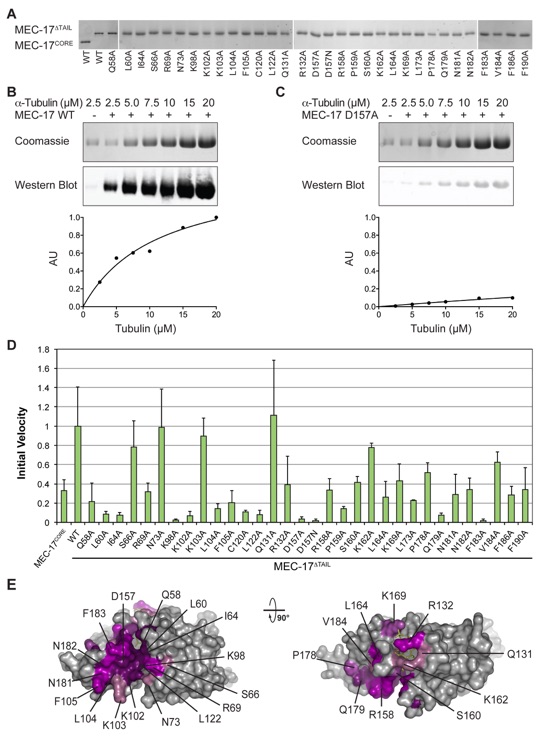
Figure 4. Mutational Analysis (a) WT and mutant forms of MEC-17 used in in vitro biochemical assays visualized on SDS-PAGE gels. (b) Representative biochemical data for wildtype MEC-17. Reaction volumes separated by SDS-PAGE stained with Coomassie brilliant blue, or immunoblotted with an acetylated K40 α-tubulin specific antibody (top). Quantification of immunoblots using an Odyssey Imaging System (bottom). (c) The same as in (b), for a catalytically dead mutant (D157A). (d) Normalized initial velocities of all tested MEC-17CORE mutants. Error bars represent standard error. All experiments were repeated at least 3 times. (e) Surface representation colored according to relative acetyltrasferase activity of alanine mutants in a gradient from light purple (>80 % activity) to dark purple (<20 % activity). A 90° rotated view is shown on the right.
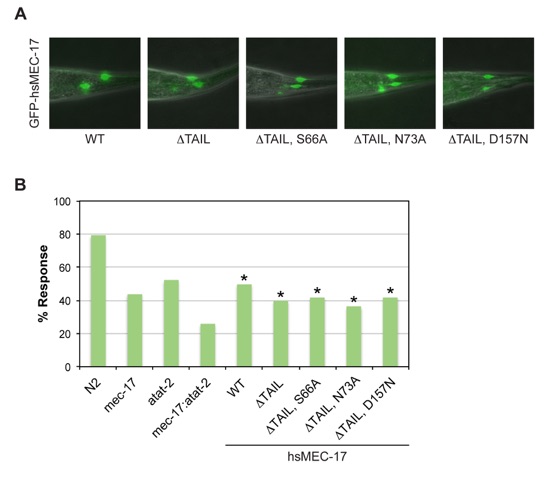
Figure 5. Functional Analysis of Human MEC-17 Variants in C. elegans. (a) Merged differential interference contrast (DIC) and fluorescent images of representative transgenic worms expressing GFP-tagged human MEC-17 variants. (b) Touch sensitivity of C. elegans strains, represented as percentage of positive responses. Asterisks indicate statistical significance based on Fisher’s Exact Test. N2 denotes the wild-type strain, mec-17 the single null mutant mec-17(ok2109) strain, atat-2 the single null mutant atat-2(ok2415) strain, and mec-17; atat-2 the double null mutant mec-17(ok2109); atat-2(ok2415) strain. Remaining strains are the mec-17; atat-2 double null mutant strain with the indicated hsMEC-17 variant present as a transgene. All hsMEC-17 variants, irrespective of catalytic activity, rescue the touch sensitivity of the double mutant.
Figures from the paper:
Coordinates:
Abstract:
Tubulin protomers undergo an extensive array of post-translational modifications to tailor microtubules to specific tasks. One such modification, the acetylation of lysine-40 of α-tubulin, located in the lumen of microtubules, is associated with stable, long-living microtubule structures. MEC-17 was recently identified as the acetyltransferase that mediates this event. We have determined the crystal structure of the catalytic core of human MEC-17 in complex with its cofactor acetyl-CoA at 1.7Å resolution. The structure reveals that the MEC-17 core adopts a canonical Gcn5-related N-acetyltransferase (GNAT) fold that is decorated with extensive surface loops. An enzymatic analysis of 33 MEC-17 surface mutants identifies hot-spot residues for catalysis and substrate recognition. A large, evolutionarily conserved hydrophobic surface patch is identified that is critical for enzymatic activity, suggesting that specificity is achieved by interactions with the α-tubulin substrate that extend outside of the modified surface loop. An analysis of MEC-17 mutants in C. elegans shows that enzymatic activity is dispensable for touch sensitivity.
California Institute of Technology
Division of Chemistry & Chemical Engineering
1200 E. California Blvd.
Pasadena, CA 91125-7200
© Copyright Hoelz Laboratory

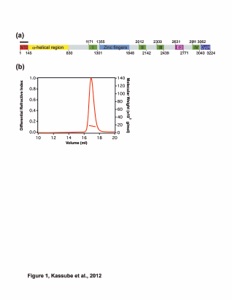
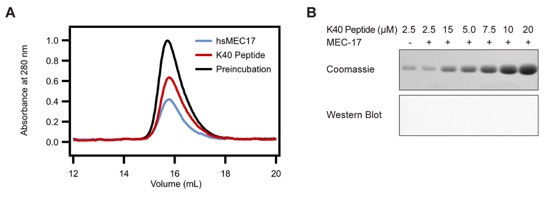
Figure S1. Substrate Peptide Analysis. (a) Purified hsMEC17CAT was mixed in excess with residues 25-49 of α-tubulin fused to SUMO and analyzed by size-exclusion chromatography. (b) Representative data from MEC-17 activity assays carried with the substrate peptide SUMO fusion in place of tubulin. Reaction volumes separated by SDS-PAGE stained with Coomassie brilliant blue, and immunoblotted with an acetylated K40 α-tubulin specific antibody. The anti-acetylated tubulin antibody recognizes an epitope within 4 residues of K40 when acetylated.
Structural and functional characterization of the α-tubulin acetyltransferase MEC-17.

Movie S1. Rotating structure of MEC-17CAT in ribbon representation.
Movie S2. Rotating structure of MEC-17CAT in surface representation.
Movie S3. Response of the N2 Bristol C. elegans strain to stroking with an eyebrow hair.
Movie S4. Response of the mec-17(ok2109); atat-2(ok2415) C. elegans strain to stroking with an eyebrow hair.
Davenport, A.M., Collins, L.N., Chiu, H., Minor, P.J., Sternberg, P.W., Hoelz, A.*
(2014). J. Mol. Biol. 426, 2605-2616