Hoelz Lab: Publications
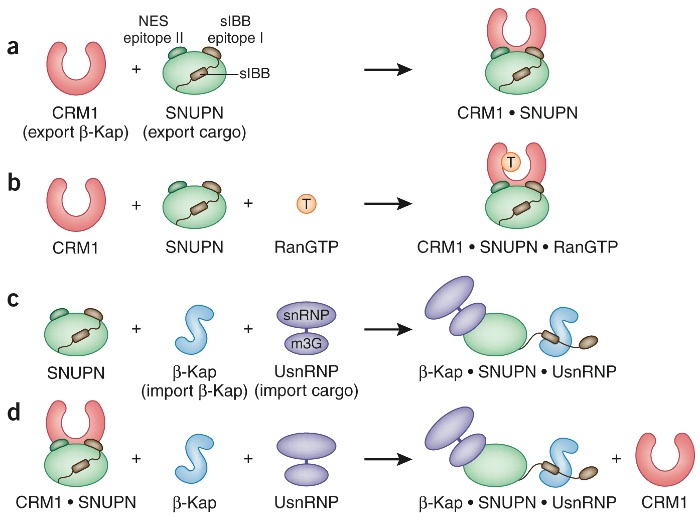
Figure 1. Assembly and disassembly of the CRM1 nuclear export complex. (a) Assembly of the binary complex between the export Kap-β CRM1 (pink) and its cargo SNUPN (green). The structure of CRM1–SNUPN reveals a bipartite CRM1 binding site, comprising the leucine-rich nuclear export sequence (NES, epitope I) and a second NES (epitope II). Epitope I falls within the SNUPN N-terminal importin-β (karyopherin-β) binding domain (sIBB, yellow). SNUPN induces a conformational change in CRM1. (b) The fully assembled export complex includes the small GTPase Ran in its GTP-bound state (orange). Dong et al. suggest that no further conformational change occurs in CRM1 upon ternary complex formation, explaining their cooperative binding. (c) SNUPN specifically imports UsnRNPs (purple). In the assembly of the Kap-β–SNUPN–UsnRNP import complex, SNUPN adopts an analogous role to an adaptor Kap-α by specifically tethering its cargo (UsnRNPs) to the import Kap-β (blue) via its sIBB. Cargo selection by SNUPN is achieved by the m3G-cap, which is generated in the final cytoplasmic maturation step of UsnRNPs. (d) The binary CRM1–SNUPN complex can be dismantled by binding of Kap-β and UsnRNP cargo owing to mutually exclusive binding sites in SNUPN.

Debler, E.W., Blobel, G., Hoelz, A. (2009). Nat. Struct. Mol. Biol. 16, 457-459.
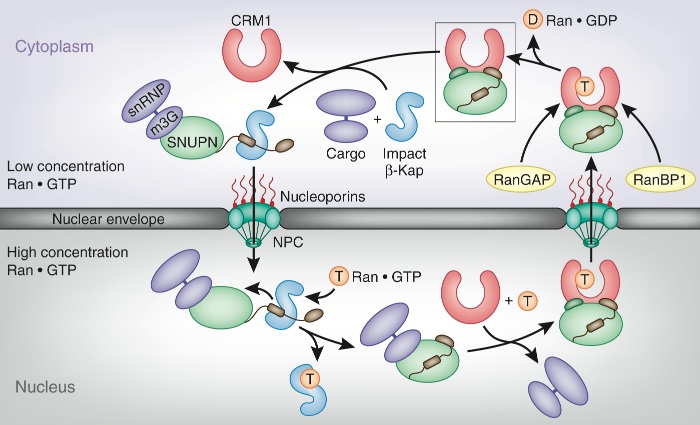
Figure 2. The SNUPN transport cycle. In the cytoplasm, assembled and matured UsnRNPs associate with SNUPN and an import Kap-β to be translocated across the nuclear envelope through the nuclear pore complex (NPC). Upon entrance into the nucleus, Ran-GTP triggers the cooperative disassembly of the import complex to yield Kap-β–Ran-GTP and SNUPN–UsnRNP. Binding of the export Kap-β CRM1 and Ran-GTP to SNUPN leads to the release of the UsnRNP cargo and the formation of the CRM1–SNUPN–Ran-GTP export complex. Upon arrival of the ternary export complex at the cytoplasmic face of the NPC, the multiple Ran-GTP binding domains of Nup358 and the single Ran-GTP binding site of soluble Ran binding protein 1 (Ran-BP1, brown) capture Ran-GTP from assembled export complexes, whereas Ran GTPase-activating protein (Ran-GAP, brown) accelerates Ran’s intrinsic GTPase activity. The concerted action of these proteins preys on Ran-GTP from the export complex, initiating its disassembly. Dong et al. find that the resulting binary CRM1–SNUPN complex can be dismantled by the addition of import Kap-β and UsnRNP cargo for a new round of nuclear import. The boxed complex refers to the structure determined by Dong et al.1.
Figures from the paper:
Abstract:
Previous structural snapshots of snurportin have provided insights into its cargo recognition and nuclear import. The structure of snurportin bound to its export factor CRM1 now reveals the molecular basis of its recycling back into the cytoplasm, illuminating general principles of nuclear export sequence recognition.
Nuclear transport comes full circle



California Institute of Technology
Division of Chemistry & Chemical Engineering
1200 E. California Blvd.
Pasadena, CA 91125-7200
© Copyright Hoelz Laboratory
