Hoelz Lab: Publications
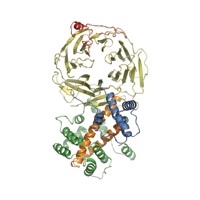
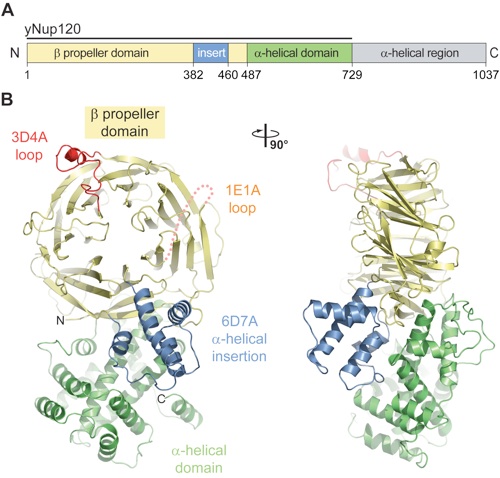
Figure 1. Structure of the S. cerevisiae Nup120 NTD. (A) Domain structure. Yellow, β propeller domain; blue, α-helical insertion in the 6D7A loop; green, α-helical domain; gray, α-helical region. The bar above the domain structure denotes the crystallized fragment. (B) Structure of the Nup120 NTD in ribbon representation, color-coded as in (A). A 90°-rotated view is shown on the right.

Seo, H.S., Ma, Y., Debler, E.W., Wacker, D., Kutik, S., Blobel, G., Hoelz, A.
(2009). Proc. Natl. Acad. Sci. USA 106, 14281-14286.
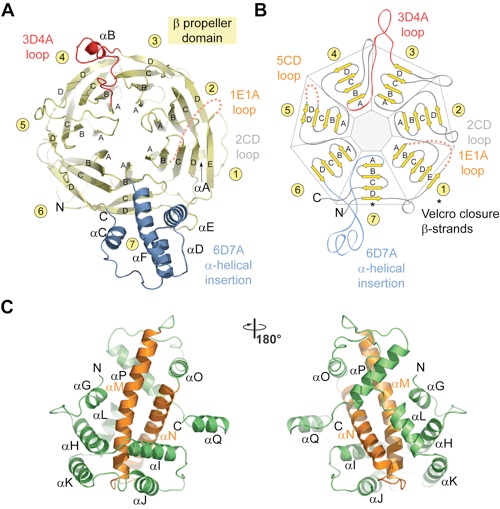
Figure 2. Structural analysis of the Nup120 NTD domains. (A) Ribbon representation of the Nup120 β propeller domain. The 7 blades of the β propeller core (yellow), the location of the disordered 1E1A loop (orange), the 3D4A loop (red), the α-helical insertion in the 6D7A loop (blue), and their secondary structure elements are indicated. (B) Schematic representation of the Nup120 β propeller domain and the locations of its various insertions. (C) Ribbon representation of the Nup120 α-helical domain. The leucine zipper–like core (orange) and the 9 surrounding α-helices (green) are indicated. A 180°-rotated view is shown on the right.
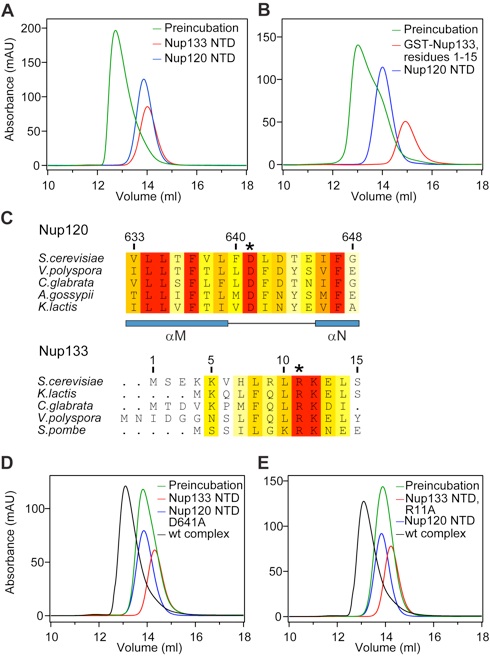
Figure 3. Nup120 NTD interacts with the 15 N-terminal residues of Nup133. (A) Gel filtration profiles of Nup120 NTD (blue), Nup133 NTD (red), and the Nup120 NTD·Nup133 NTD complex (green). (B) Gel filtration profiles of Nup120 NTD (blue), the 15 N-terminal residues of Nup133 fused to GST (red), and their complex (green). All proteins were injected at approximately the same concentrations. (C) The invariant Asp-641 of Nup120 and Arg-11 of Nup133 are key residues for complex formation. The location of Asp-641 and Arg-11 are indicated by asterisks in multispecies sequence alignments. (D) Gel filtration profiles of Nup120 NTD D641A mutant (blue) and the Nup133 NTD (red), and the elution profile resulting from incubation of the 2 proteins before injection (green). (E) Gel filtration profiles of Nup120 NTD (blue) and the Nup133 NTD R11A mutant (red), and the elution profile resulting from incubation of the 2 proteins before injection (green). As a reference, the gel filtration profile of the wild-type Nup120 NTD·Nup133 NTD is indicated in black.
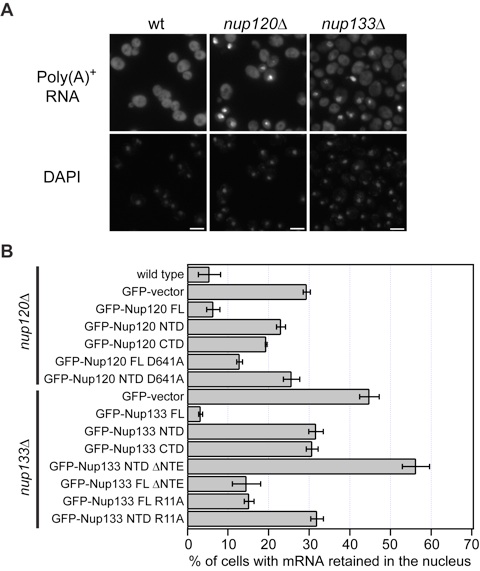
Figure 4. Physiological relevance of the Nup120–Nup133 interaction. (A) Detection of poly(A)+ mRNA using an Alexa-647 labeled 50-mer oligo dT FISH probe (Top). Wild-type cells (Left) display a diffuse FISH signal, while nup120Δ (Middle) and nup133Δ (Right) cells yield strong nuclear signals that coincide with DAPI staining, consistent with poly(A)+ mRNA retention inside the nucleus. (B) Quantitation of nuclear poly(A)+ mRNA retention in nup120Δ and nup133Δ yeast strains complemented with various Nup120 and Nup133 variants. The percentages refer to the fraction of cells that displayed marked nuclear staining and are derived from 3 independent experiments.
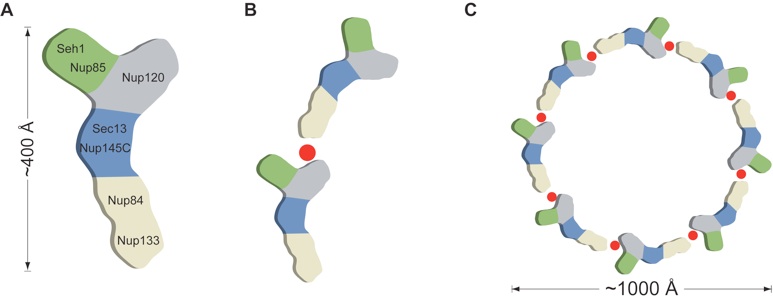
Figure 5. Model for the ring formation of the Nup84 complex. (A) Schematic representation of the heptameric complex and the approximate localization of its 7 nups (19). (B) The interaction of Nup120 and Nup133 suggests the intermolecular interaction between 2 heptamers in a head-to-tail fashion mediated by a short stretch at the very N terminus of an extended unstructured region of Nup133. (C) Eight heptamers are arranged in a closed ring with a diameter of ≈1,000 Å in accordance with the NPC dimensions determined by cryo-electron microscopy (8).
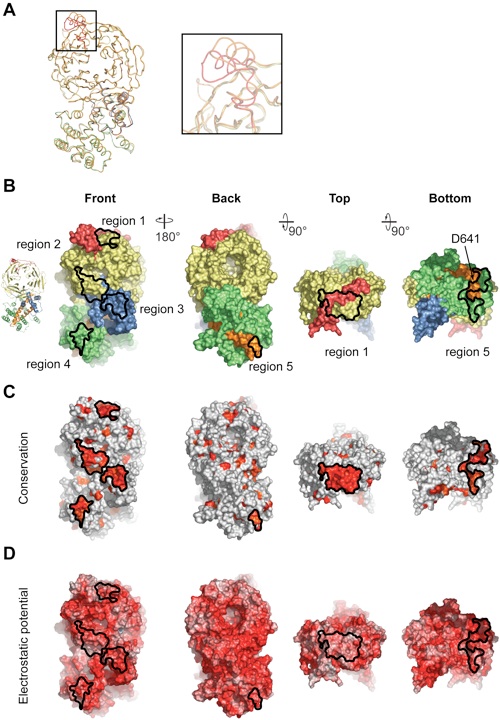
Figure S1.Structural properties of the Nup120 NTD. (A) Comparison of the Nup120 structures from the wild-type and S207C mutant proteins. Coil representations of the superposition of the S207C mutant Nup120 (colored according to Fig. 1) and the wild-type Nup120 structure (orange). The inset highlights significant structural changes in the 3D4A loop. (B–D) Surface properties of the Nup120 NTD. (B) Surface representation colored according to the participation of the various domains as in Fig. 1B. The two left panels show the front and back views of the structure; the two right panels show the views from the top and bottom. (C) Surface representation colored according to a multispecies sequence alignment (Fig. S2). The conservation at each position is mapped onto the surface and is shaded in a color gradient from light yellow (40% similarity) to dark red (100% identity). (D) Surface representation colored according to the electrostatic potential. The electrostatic potential is plotted onto the surface and colored in a gradient from red (–10 kBT/e) to blue (+10 kBT/e). The orientation of all surface representations is identical in each column. As a reference, black lines encircle the 5 conserved surface patches.
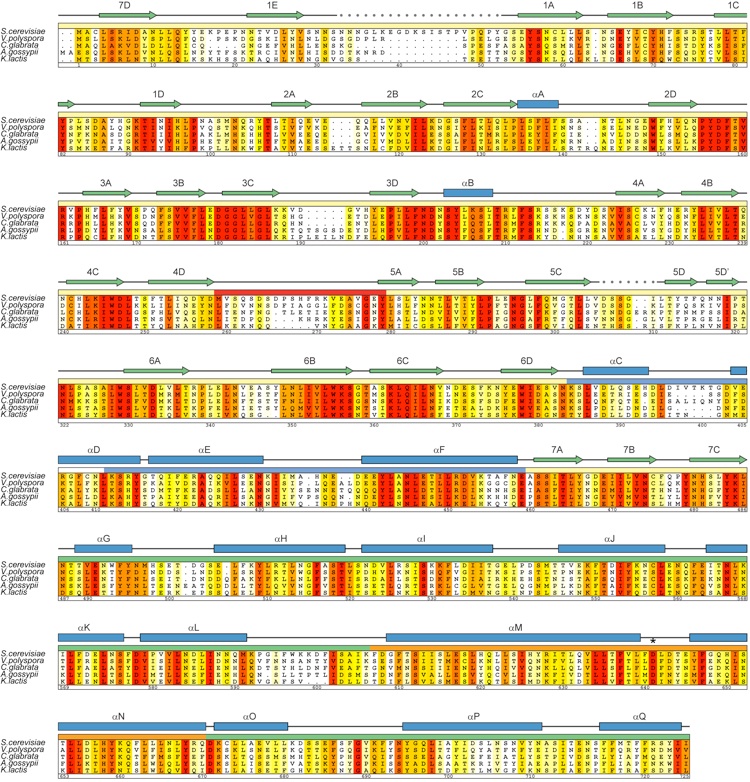
Figure S2.Multispecies sequence alignment of Nup120 homologs. The numbering below the alignment is relative to S. cerevisiae Nup120. The overall sequence conservation at each position is shaded in a color gradient from yellow (40% similarity) to dark red (100% identity) using the Blosum62 weighting algorithm. The secondary structure is indicated above the sequence as blue boxes (alpha helices), green arrows (strands), gray lines (coil regions), and gray dots (disordered residues). The position of the invariant Asp-641 that is critical for the interaction with Nup133 is indicated by an asterisk.
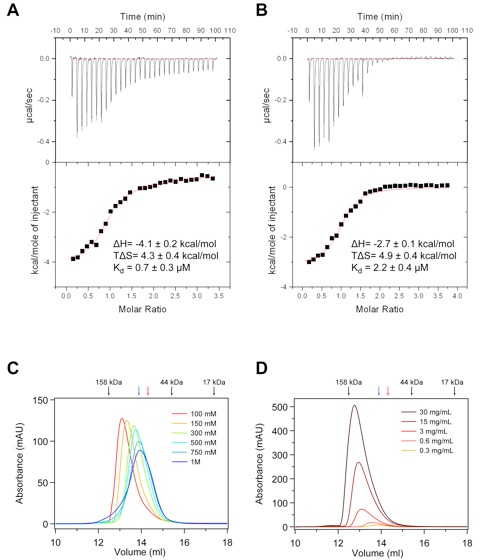
Figure S4.Biochemical characterization of the Nup120 –Nup133 interaction. (A and B) ITC analysis of the Nup120ΔNup133 complex. The Nup120 NTD was titrated against (A) the Nup133 NTD and (B) a Nup133 peptide comprising residues 1–15. The dissociation constant (Kd), the binding enthalpy (ΔH), and the entropy (TΔS) were derived by curve fitting using the single-site model. The binding constant of the Nup133 peptide is decreased only slightly with respect to the entire Nup133 NTD, suggesting that the major Nup120 binding site comprises the first 15 residues of Nup133. (C) Salt dependence of the Nup120 NTD–Nup133 NTD interaction. Gel filtration profiles of the Nup120 NTDΔNup133 NTD complex (5 mg/mL) at various salt concentrations. The Nup120 NTDΔNup133 NTD complex eluted with an apparent molecular mass of ~120 kDa (calculated molecular mass of the complex, ~140 kDa) in a buffer containing 100 mM NaCl. The apparent molecular mass of the peak decreased to ~80 kDa when examined in a buffer containing 1 M NaCl. (D) Concentration dependence of the Nup120 NTD–Nup133 NTD interaction. Proteins were injected at the indicated concentrations. Whereas the complex eluted with an apparent molecular mass of ~135 kDa at 30 mg/mL, the apparent molecular mass of the peak decreased to ~80 kDa when examined at 0.3 mg/mL. As a reference, the elution positions of molecular weight standards, Nup120 NTD (blue), and Nup133 NTD (red) are indicated. The Nup120ΔNup133 complex is dynamic and governed by electrostatic interactions.
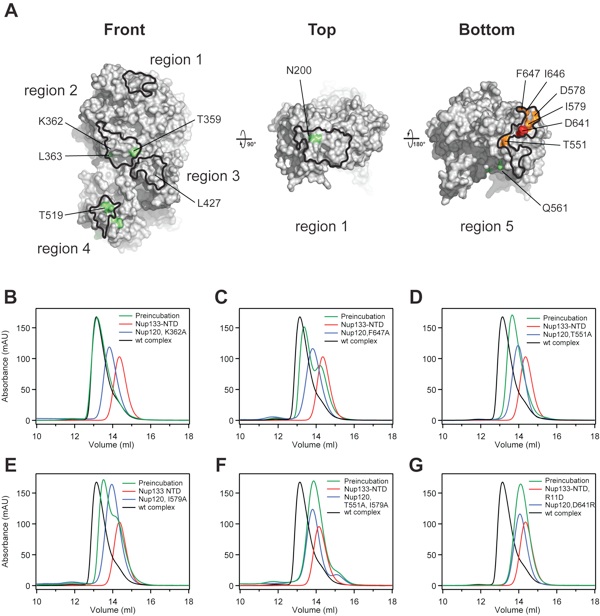
Figure S5.Alanine scanning mutagenesis of the Nup120–Nup133 interaction. (A) Surface representation of the Nup120 NTD with the highlighted 5 regions identified by surface conservation analysis (Fig. S1C). Nup120 residues of the 5 surface patches that have no detectable effect (green), have only a moderate effect (orange), and are crucial (red) for complex formation with Nup133, as judged by size-exclusion chromatography, are indicated and mapped to the surface. (B–G) Representative gel filtration profiles for Nup120 NTD mutants that form a complex with the Nup133 NTD that is indistinguishable from the wild-type complex (B), have a small detectable effect (C), have a moderate effect (D and E), or abolish complex formation (F and G). Note that the 2 Nup120 NTD mutations in region 5, T551A and I579A, which individually have only moderate effects on complex formation, abolish complex formation when combined. Gel filtration profiles of the Nup120 NTD mutants (blue), the Nup133 NTD (red), and the elution profiles resulting from incubation of the 2 proteins before injection (green) are indicated. As a reference, the elution profile of the wild-type Nup120 NTDΔNup133 NTD complex is shown in black. Proteins were injected at comparable concentrations. The results of all analyzed mutants are presented in Table S2.
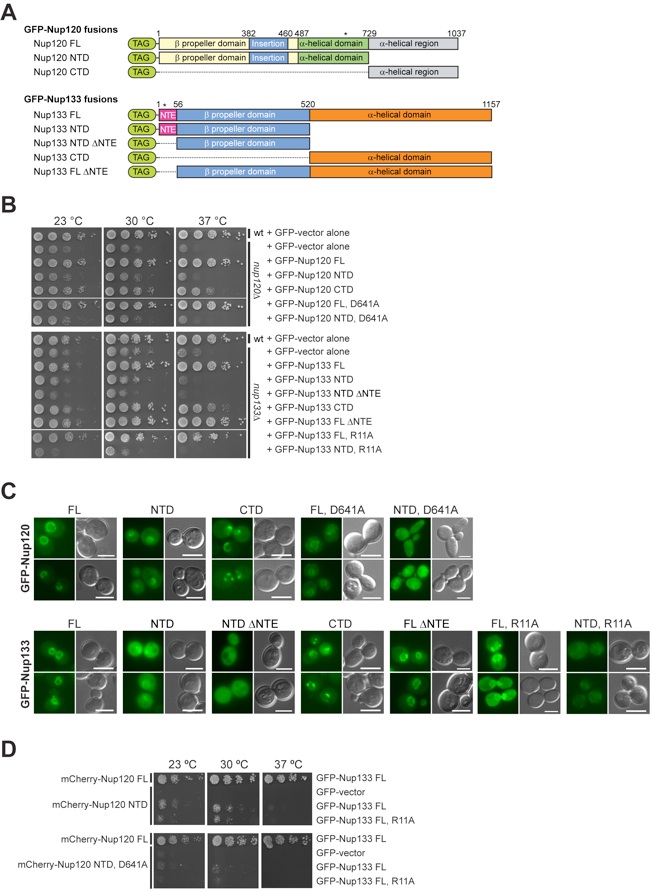
Figure S6.Physiological relevance of the Nup120 –Nup133 interaction. (A) The domain organization of the Nup120 and Nup133 GFP-fusion proteins is indicated. The domain organization of Nup120 is based on the structural data. For Nup133, the unstructured NTE (purple), the N-terminal beta propeller domain (blue), and the C-terminal alpha-helical domain (orange) are indicated. (B and C) Yeast growth assay (B) and in vivo localization (C) performed using either nup120Δ or nup133Δ cells complemented with the constructs described in (A). (D) Yeast growth analysis of Nup120 and Nup133 variants in nup120Δnup133Δ yeast cells.
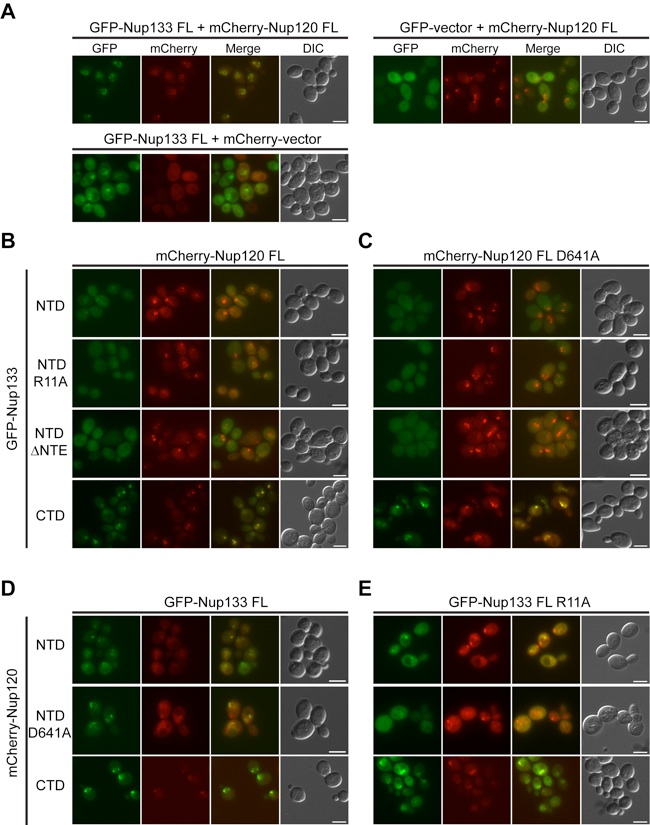
Figure S7.Codependence of the subcellular localization of Nup120 and Nup133. Fluorescence microscopy analysis of nup120Δnup133Δ yeast cells complemented with various GFP-Nup133 and mCherry-Nup120 variants according to Fig. S6 A. (A) While coexpression of fluorescence-tagged full-length Nup120 and Nup133 proteins results in a wild-type-like continuous rim staining, deletion of either of the 2 proteins leads to a punctate rim staining. (B–E) Analysis of nup120Δnup133Δ yeast cells complemented with full-length mCherry-Nup120 and various GFP-Nup133 variants (B), full-length mCherry-Nup120 carrying the D641A mutation and various GFP-Nup133 variants (C), full-length GFP-Nup133 and various mCherry-Nup120 variants (D), and full-length GFP-Nup133 carrying the R11A mutation and various mCherry-Nup120 variants (E).
PDB coordinates (link to PDB site) - 3F7F, 3H7N
PDB coordinates - 3F7F (.pdb), 3H7N (.pdb)
Structure factors - 3F7F (.txt), 3H7N (.txt)
Figures from the paper:
Coordinates:
Abstract:
The Nup84 complex constitutes a key building block in the nuclear pore complex (NPC). Here we present the crystal structure of one of its 7 components, Nup120, which reveals a beta propeller and an alpha-helical domain representing a novel fold. We discovered a previously unidentified interaction of Nup120 with Nup133 and confirmed the physiological relevance in vivo. As mapping of the individual components in the Nup84 complex places Nup120 and Nup133 at opposite ends of the heptamer, our findings indicate a head-to-tail arrangement of elongated Nup84 complexes into a ring structure, consistent with a fence-like coat for the nuclear pore membrane. The attachment site for Nup133 lies at the very end of an extended unstructured region, which allows for flexibility in the diameter of the Nup84 complex ring. These results illuminate important roles of terminal unstructured segments in nucleoporins for the architecture, function, and assembly of the NPC.
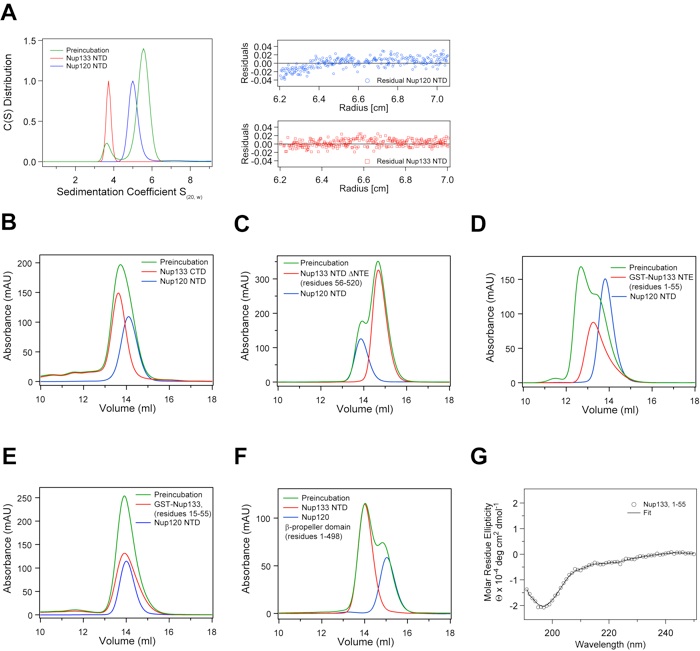
Figure S3.Biochemical and biophysical analyses of the interaction between Nup120 and Nup133. (A) Sedimentation velocity analysis of the Nup120 NTD–Nup133 NTD interaction. Sedimentation coefficient distributions of the Nup120 NTD (blue), of the Nup133 NTD (red), and the Nup120ΔNup133 complex (green). The large green peak indicates complex formation, while the small green peak corresponds to excess unbound Nup133 NTD. The molecular masses for Nup120 and Nup133 have been determined to 82 +/– 8 (calculated 84.2 kDa) and 54 +/– 2 kDa (55.7 kDa), respectively, corresponding to monomers in solution. The precise determination of the molecular mass of the Nup120ΔNup133 complex is not possible, due to the presence of a second peak. (B–F) Further biochemical analysis of the Nup120–Nup133 interaction by size-exclusion chromatography. (B) The Nup133 CTD (residues 520 –1157) and (C) the Nup133 NTD ΔNTE (residues 56 –520), form no detectable complex with the Nup120 NTD. (D) The Nup133 NTE, residues 1–55, fused to GST forms a complex with Nup120 NTD. (E) The GST-tagged Nup133 fragment comprising residues 15–55 fails to interact with Nup120 NTD. (F) The Nup120 propeller domain, residues 1– 498, is incapable of forming a complex with the Nup133 NTD. Gel filtration profiles of Nup120 fragments (blue), Nup133 fragments (red), and the elution profiles resulting from incubation of the 2 proteins before injection (green) are indicated. Proteins were injected at comparable concentrations. (G) The CD spectrum of the Nup133 NTE, residues 1–55, reveals a random coil conformation for this region (23).

Structural and functional analysis of Nup120 suggests ring formation of the Nup84 complex.
California Institute of Technology
Division of Chemistry & Chemical Engineering
1200 E. California Blvd.
Pasadena, CA 91125-7200
© Copyright Hoelz Laboratory
