Hoelz Lab: Publications
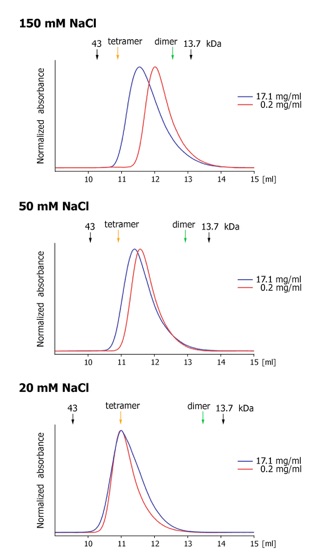
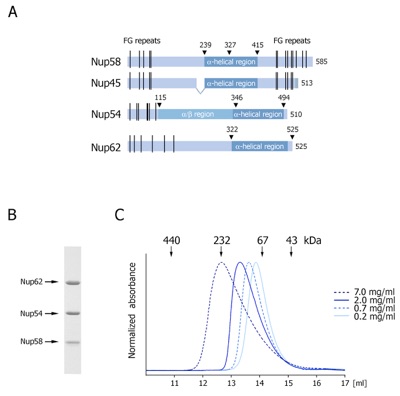
Figure 1. Organization and dynamic behavior of the Nup62 complex. (A) Domain structures of Nup58, Nup45, Nup54 and Nup62. The alpha-helical regions (dark blue), unstructured regions (light blue), FG-repeats (black lines), and the numbering of the Nup62 complex fragments are indicated. (B) Coomassie-stained SDS-PAGE gel of the purified Nup62 core complex. (C) Gel filtration profiles of the minimal Nup62 complex at the indicated protein concentrations. The elution positions for molecular weight standards are shown. All profiles were obtained in a buffer containing 150 mM NaCl.

Melcak, I., Hoelz, A.*, Blobel, G.* (2007). Science 315, 1729-1732.
* Corresponding authors
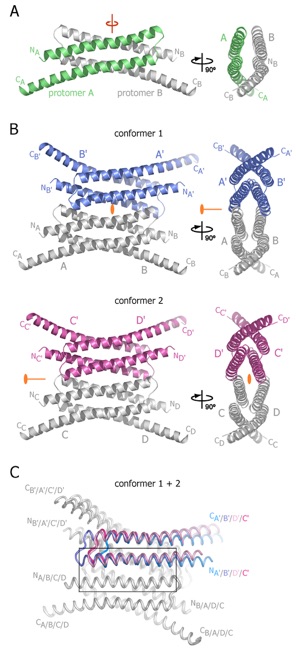
Figure 2. Structures of tetrameric Nup58/45 assemblies from the tetragonal crystal form. (A) Ribbon representation of a Nup58/45 dimer, showing protomer A in green and protomer B in gray; a 90 rotated view is shown on the right. A pseudo-two-fold axis (red) is indicated. (B) Ribbon representations of the two tetrameric conformers, indicating the location of the crystallographic two-fold axes (orange), which run differently through the two tetramers. A 90 rotated view is shown on the right. The different coloring of dimers (blue, conformer 1; purple, conformer 2) illustrate the alternative tetrameric configurations of the two conformers. Symmetry-related protomers are indicated. Conformers 1 and 2 are constructed by the protomers A and B, C and D, and their symmetry-related protomers A’ and B’ and C’ and D’, respectively. (C) Superposition of the two tetrameric Nup58/45 conformers. The unique protomers of the two tetrameric assemblies are superimposed onto protomer A of conformer 1 to highlight the lateral shift between the different conformers. For clarity, only one protomer of each superposition is colored. The N- and C-termini are labeled according to (B). The inset is expanded in Figure 3.
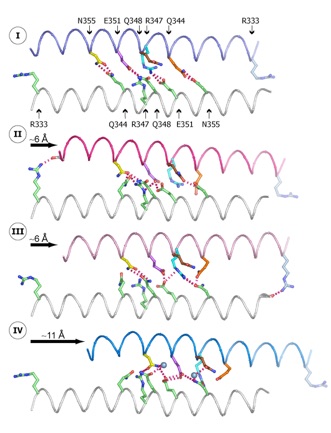
Figure 3. Molecular details of the intermolecular sliding of two Nup58/45 dimers. The direction and the approximate sliding distance are indicated by black arrows. States I and IV are derived from conformer 1, and states II and III are derived from conformer 2. The helices are colored and numbered according to Figure 2, and critical interface residues are labeled and individually colored in all four states according to state I (top). For clarity, only two of the N-helix pairs that generate the tetramerization interface are shown. The electrostatic interactions between two sliding N-helices are represented by red dashed lines, and water molecules are visualized as blue spheres.
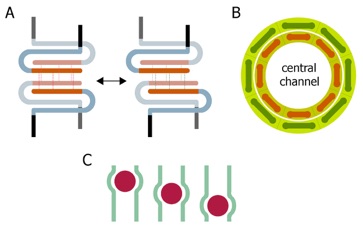
Figure 4. Model of pore dilation by intermolecular sliding of Nup58/45 tetramers. (A) Schematic representation of the Nup58/45 sliding module. The four N-helices that generate the tetramerization interface (orange), the C-helices (light blue), and the C-terminal FG-repeats (black) are indicated. The sliding of the Nup58/45 dimer surfaces formed by the N-helices is facilitated by alternative hydrogen bond network (red and green thin lines). Because the C-helices with the attached FG-repeats are likely to project to the inside of the central channel, the sliding N-helices would face in the opposite direction. (B) Schematic representation of the central channel shown along the nucleocytoplasmic axis. Eight Nup58/45 tetrameric assemblies are circularly arranged to form a ring (red bars). The sliding of two Nup58/45 dimers against each other results in an overall extension of the Nup58/45 tetramer (green bars) that, in turn, causes the dilation of the central channel of the NPC. (C) Localized changes in channel diameter by alpha-helical sliding in response to cargo (red spheres) transport across the central channel (green).
Figure S1. Gel filtration profiles of Nup58/45327-399 at the indicated protein and salt concentrations. The elution positions for molecular weight standards are shown, and the predicted dimer and tetramer positions are indicated.
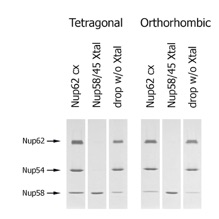
Figure S2. Analysis of the crystal content. SDS-PAGE gel illustrating the content of the tetragonal and orthorhombic crystals and the remaining solution of the crystallization drops. Both crystal forms only contain Nup58/45, while Nup62, Nup54, and excess Nup58/45 remain un-proteolyzed in the drop.
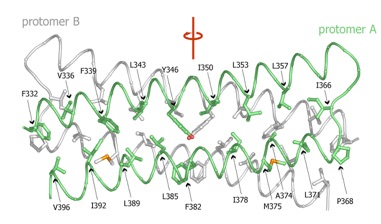
Figure S3. Molecular details of the dimerization interface. The C traces of protomer A (green) and B (gray) are shown in coil representation, and the interface residues are shown in ball-and-stick representation. A pseudo-two-fold axis (red) runs vertically through the dimerization interface. For clarity, only residues of protomer A are labeled.
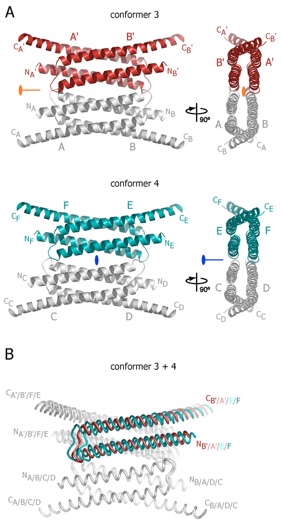
Figure S4. Structures of tetrameric Nup58/45 assemblies from the orthorhombic crystal form. (A) Ribbon representations of the two tetrameric Nup58/45 assemblies found in the orthorhombic crystal form. The asymmetric unit of the crystal contains 3 and 4 Nup58/45 tetramers of conformer 3 (dark red) and conformer 4 (teal), respectively. 90 rotated views are shown on the right. The location of the crystallographic two-fold axis (orange) in conformer 3, the non-crystallographic two-fold axis (blue) in conformer 4, and symmetry-related protomers are indicated. Conformer 3 and 4 are formed by protomers A and B, and their symmetry related protomers A’ and B’, and protomers C, D, E, and F, respectively. (B) Superposition of the two tetrameric Nup58/45 conformers. The unique protomers of the two tetrameric assemblies are superimposed onto protomer A of conformer 1 to highlight the lateral shift between the different conformers. For clarity, only one protomer of each superposition is colored. The N- and C-termini are labeled according to (A).
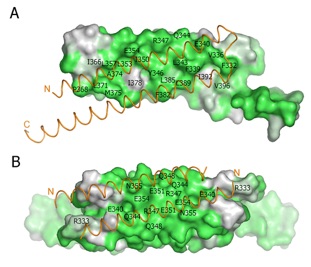
Figure S5. Surface conservation of vertebrate Nup58/45 homologs. (A, B) Surface representations of the oligomerization interfaces. The surface of the dimerization interface of one protomer (A) and the tetramerization interface of the dimer subunit (B) are shown. The conservation of the surfaces is indicated by a color gradient from white (60 % identity) to dark green (100 % identity). The associated -helices are depicted as orange coil representations.
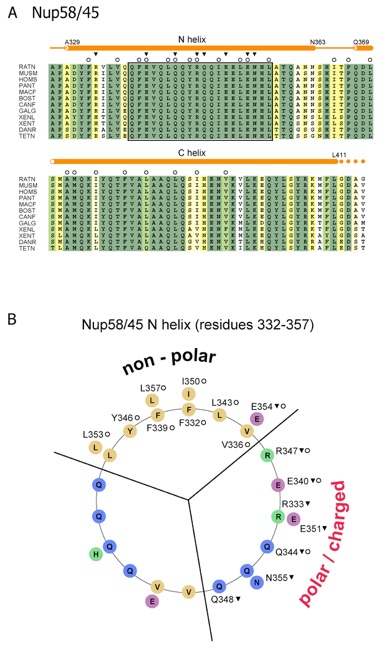
Figure S6. Sequence analysis of vertebrate Nup58/45. (A) Multiple sequence alignment of Nup58/45 from different species. The secondary structure elements are indicated as orange cylinders (-helices), orange lines (coil regions), and solid orange circles (disordered residues). Numbering is relative to Rattus norvegicus Nup58. The sequence conservation is shown in a color gradient from light yellow (60 % identity) to dark green (100 % identity). The participation of various residues in the formation of the two interfaces is indicated by open circles (dimer interface) and solid triangles (tetramer interface). The conserved region of the tetramerization interface is boxed. The abbreviations for the sequences are: Rattus norvegicus (RATN), Mus musculus (MUSM), Homo sapiens (HOMS), Pan troglodytes (PANT), Macaca fascicularis (MACF), Bos taurus (BOST), Canis familiaris (CANF), Gallus gallus (GALG), Xenopus laevis (XENL), Xenopus tropicalis (XENT), Danio rerio (DANR), and Tetraodon nigroviridis (TETN). (B) Helical wheel representation illustrating the electrostatic character of the Nup58/45 N-helix (residues 332 to 355). The involvement of the residues in the formation of the various interfaces is labeled according to (A).
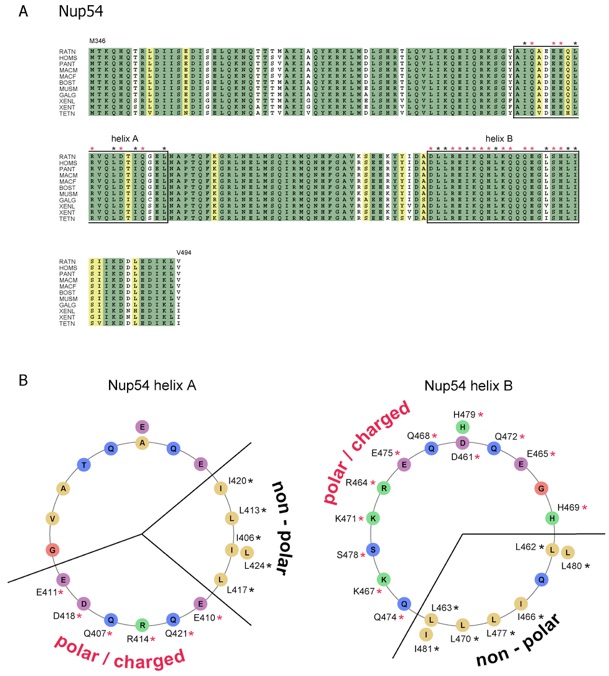
Figure S7. Sequence analysis of vertebrate Nup54. (A) Multiple sequence alignment of Nup54 from different species. Numbering is relative to Rattus norvegicus Nup54. The alignment is color-coded according to Figure S6. The abbreviations for the sequences are: Rattus norvegicus (RATN), Homo sapiens (HOMS), Pan troglodytes (PANT), Macaca mulatta (MACM), Macaca fascicularis (MACF), Bos taurus (BOST), Mus musculus (MUSM), Gallus gallus (GALG), Xenopus laevis (XENL), Xenopus tropicalis (XENT), and Tetraodon nigroviridis (TETN). The potential involvement of predicted helical residues in non-polar (black stars) and electrostatic interactions (red stars) is indicated. Residues arranged in the helical wheel representation (B) are boxed. (B) Helical wheel representation illustrating the electrostatic character of two Nup54 -helices that potentially form sliding regions similar to Nup58/45. The involvement of the residues in the formation of the non-polar and electrostatic interfaces is indicated and labeled according to (A).
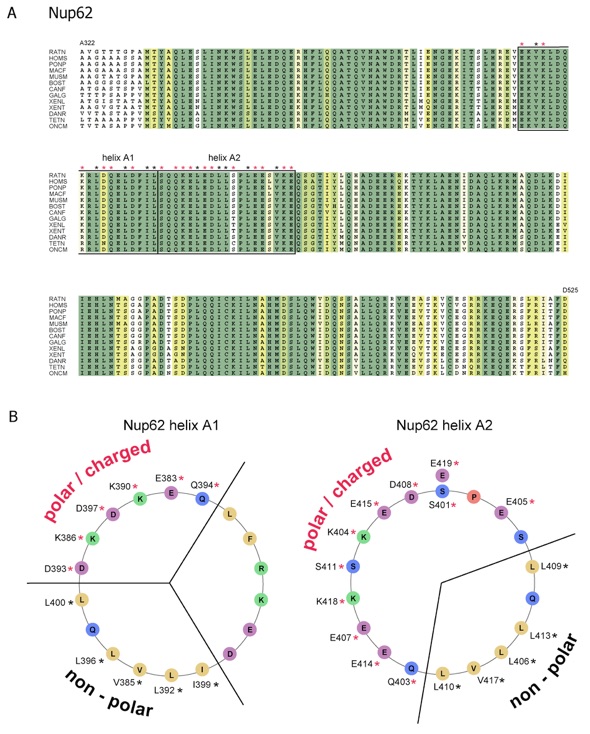
Figure S8. Sequence analysis of vertebrate Nup62. (A) Multiple sequence alignment of Nup62 from different species. Numbering is relative to Rattus norvegicus Nup62. The alignment is color-coded and labeled according to Figure S6. The abbreviations for the sequences are: Rattus norvegicus (RATN), Homo sapiens (HOMS), Pongo pygmaeus (PONP), Macaca fascicularis (MACF), Mus musculus (MUSM), Bos taurus (BOST), Canis familiaris (CANF), Gallus gallus (GALG), Xenopus laevis (XENL), Xenopus tropicalis (XENT), Danio rerio (DANR), Tetraodon nigroviridis (TETN), and Oncorhynchus mykiss (ONCM). (B) Helical wheel representation illustrating the electrostatic character of two Nup62 -helices potentially form sliding regions similar to Nup58/45. The involvement of the residues in the formation of the non-polar and electrostatic interfaces is indicated and labeled according to (A).
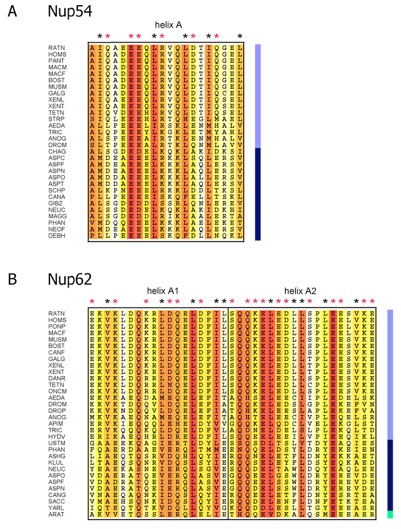
Figure S9. Sequence alignments of predicted sliding -helices of Nup54 and Nup62 across eukaryotes. (A) Multi-species sequence alignment of helix A of Nup54 (see Fig. S7) and (B) of helix A1 and A2 of Nup62 (see fig. S8). Numbering is relative to Rattus norvegicus Nup54 and Nup62. The alignment is color-coded using the BLOSUM 62 matrix from white (40 % similarity) to red (100 % similarity), and labeled according to figs. S7-8. Color bar represents metazoans (light blue), fungi (dark blue) and plants (green). The abbreviations for the sequences are: Aedes aegypti (AEDA), Anopheles gambiae (ANOG), Apis mellifera (APIM), Arabidopsis thaliana (ARAT), Ashbya gossypii (ASHG), Aspergillus clavatus (ASPC), Aspergillus fumigatus (ASPF), Aspergillus nidulans (ASPN), Aspergillus oryzae (ASPO), Aspergillus terreus (ASPT), Bos taurus (BOST), Candida albicans (CANA), Candida glabrata (CANG), Canis familiaris (CANF), Chaetomium globosum (CHAG), Danio rerio (DANR), Debaryomyces hansenii (DEBH), Drosophila melanogaster (DROM), Drosophila pseudoobscura (DROP), Gallus gallus (GALG), Giberella zeae (GIBZ), Homo sapiens (HOMS), Hydra vulgaris (HUDV), Kluyveromyces lactis (KLUL), Macaca fascicularis (MACF), Macaca mulatta (MACM), Magnaporthe grisea (MAGG), Mus musculus (MUSM), Neosartorya fischeri (NEOF), Neurospora crassa (NEUC), Oncorhynchus mykiss (ONCM), Pan troglodytes (PANT), Phaeosphaeria nodorum (PHAN), Pongo pygmaeus (PONP), Rattus norvegicus (RATN), Saccharomyces cerevisiae (SACC), Schizosaccharomyces pombe (SCHP), Strongylocentrotus purpuratus (STRP), Tetraodon nigroviridis (TETN), Tribolium castaneum (TRIC), Ustilago maydis (USTM), Xenopus laevis (XENL), Xenopus tropicalis (XENT) and Yarrowia lipolytica (YARL).

Figures from the paper:
Coordinates:
Abstract:
The nucleoporins Nup58 and Nup45 are part of the central transport channel of the nuclear pore complex, which is thought to have a flexible diameter. In the crystal structure of an alpha-helical region of mammalian Nup58/45, we identified distinct tetramers, each consisting of two antiparallel hairpin dimers. The intradimeric interface is hydrophobic, whereas dimer-dimer association occurs through large hydrophilic residues. These residues are laterally displaced in various tetramer conformations, which suggests an intermolecular sliding by 11 angstroms. We propose that circumferential sliding plays a role in adjusting the diameter of the central transport channel.
Structure of Nup58/45 suggests flexible nuclear pore diameter by intermolecular sliding.
California Institute of Technology
Division of Chemistry & Chemical Engineering
1200 E. California Blvd.
Pasadena, CA 91125-7200
© Copyright Hoelz Laboratory
