Hoelz Lab: Publications
Figure 1. Biochemical analysis of mammalian and chimeric Nup98 assemblies. (a) Schematic architecture of the NPC (left panel). The cylindrical symmetric core (orange) is decorated with cytoplasmic filaments (cyan) and a nuclear basket (magenta) and anchored in the nuclear envelope by integral pore membrane proteins (POMs, green). Natively unfolded FG repeats of a number of nups make up the transport barrier in the central channel and are indicated by a transparent plug. Schematic diagram of the cytoplasmic filament interaction network of the yeast and human NPCs (right panel) as discussed in the text. (b) Domain organization of human Nup88 and the yeast homolog Nup82, mouse Nup98, and yeast Nup159. For hNup88 and yNup82, the NTD (blue) and the C-terminal α-helical domain (dark gray) are indicated. For mNup98, GLFG repeat regions (gray), the GLEBS (dark gray), the unstructured region (gray), the autoproteolytic and NPC-targeting domain (APD, green), and the C-terminal extension (termed 6-kDa fragment in the human protein) (gray) are indicated. For Nup159, the NTD (dark gray), the FG repeat region (gray), the unstructured region (gray), the dynein light chain-interacting domain (DID, dark gray), the C-terminal α-helical region (dark gray), and the tail region (T, red) are indicated. The blue bar represents the domain boundaries of the human Nup88 NTD used for interaction studies. The black bars above the domain structures denote the crystallized fragments. (c) Multiangle light-scattering (MALS) analysis of the mammalian mNup98APD·hNup88NTD heterodimer. The differential refractive indices of mNup98APD (red), hNup88NTD (blue), and mNup98APD·hNup88NTD (green) are plotted against the elution volumes from a Superdex 200 10/300 GL gel-filtration column (GE Healthcare) and are overlaid with the determined molecular masses for the selected peaks. (d) MALS analysis of the chimeric mNup98APD·yNup82NTD ·yNup159T heterotrimer. The differential refractive indices of mNup98APD (red), yNup82NTD·yNup159T (blue), and mNup98APD·yNup82NTD·yNup159T (green) are plotted against the elution volumes from a Superdex 200 10/300 GL gel-filtration column (GE Healthcare) and are overlaid with the determined molecular masses for the selected peaks.

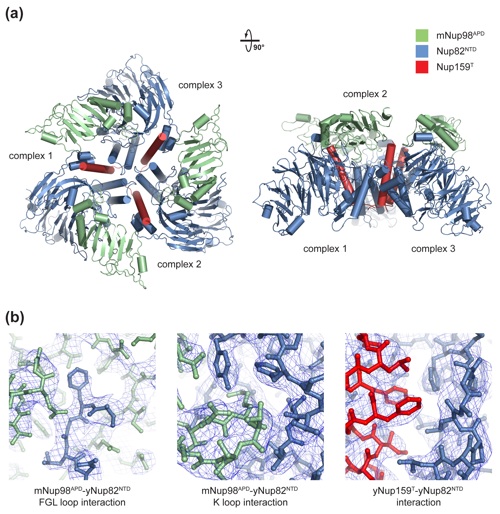
Figure 2. Structural overview of the heterotrimer. (a) Cartoon representation of the mNup98APD·yNup82NTD·yNup159T complex, showing the yNup82NTD in blue with various noncanonical insertions highlighted in yellow (3D4A or FGL loop), gray (4CD), and orange (6CD). The mNup98APD is displayed in green with the β6–αB connector (K loop) colored magenta. yNup159T is shown in red. A 180° rotated view is shown on the right. (b) Cartoon representation of mNup98APD·yNup82NTD·yNup159T, rotated by 90° with respect to (a), left panel. The seven blades of the β propeller are numbered. (c) Schematic representation of the architectures of the three domains in the mNup98APD·yNup82NTD ·yNup159T heterotrimer and their interaction. Prominent insertions and secondary structure elements are labeled. The asterisk denotes the N-terminal region that links blades 1 and 7.
Figure 3. Structural comparison of Nup98 complexes. Superposition of mNup98APD·yNup82NTD and hNup98APD·hNup986 kDa complexes. For clarity, only the FGL loop of yNup82NTD is shown (stick representation). The FGL loop and the corresponding YGL segment of hNup986 kDa bind to overlapping sites on Nup98APD as shown on the right.
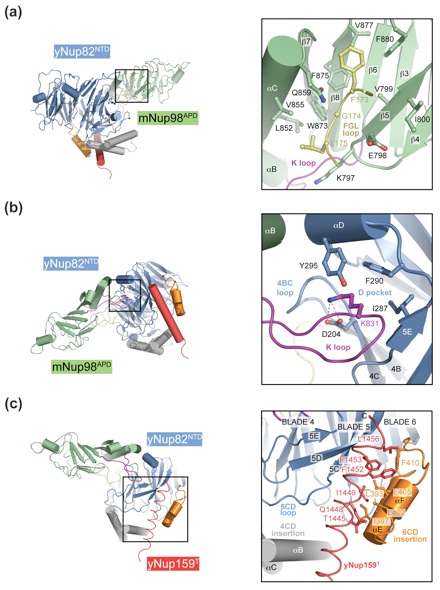
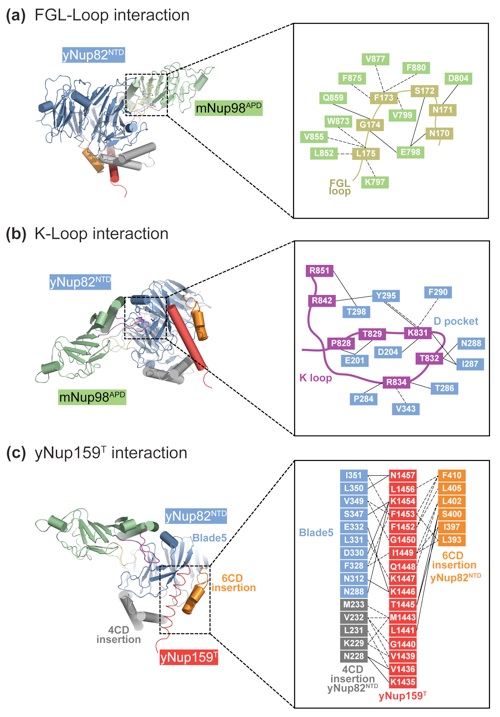
Figure 4. Interfaces in the heterotrimer. (a) Detailed view of the interaction of the extended yNup82NTD FGL loop (yellow) that binds to the hydrophobic catalytic groove of mNup98APD between helix αB and strand β5. (b) Close-up view of the interaction between the tip of the mNup98APD K loop and its association with the yNup82NTD D pocket. A key salt bridge is formed between the K loop K831 and the D pocket D204. (c) Close-up view of the interaction between yNup159T and yNup82NTD. The Nup159T binds to a hydrophobic groove in yNup82NTD β propeller that is formed by the noncanonical 4CD and 6CD insertions and blade 5. As a reference, ribbon representations of the heterotrimer are shown in the left panels. The black insets correspond to the magnified regions seen in the right panels.
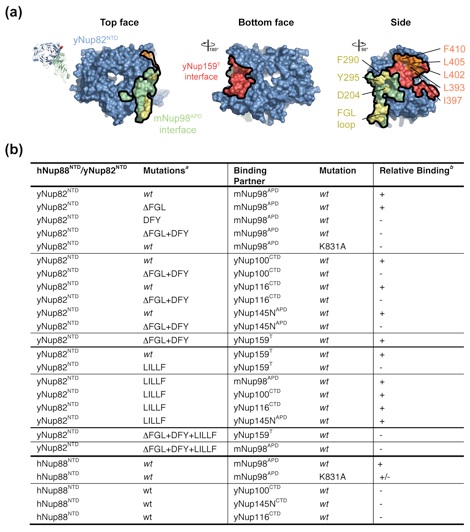
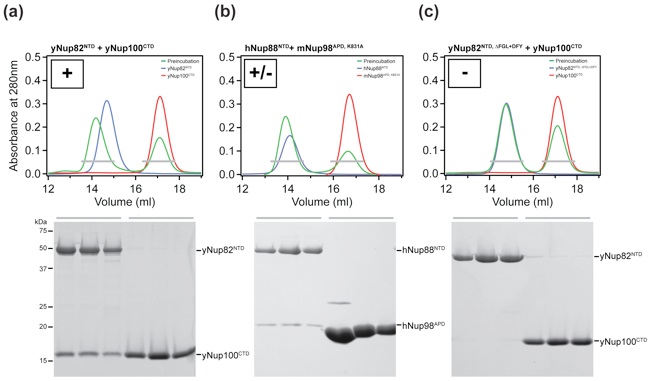
Figure 5. Biochemical analysis of the interactions in the heterotrimer. (a) Surface rendition of yNup82NTD. The mNup98APD and Nup159T binding sites are colored green and red, respectively. The positions of the DFY and the ΔFGL loop mutations that disrupt mNup98APD binding and of the LILLF mutation that abolishes the yNup159T interaction are indicated in yellow and orange, respectively. As a reference, the ribbon representation of the heterotrimer is shown on the left. (b) Interaction table summarizing the results of the mutational analysis. Binding is scored as wild type (+), intermediate (+/−), or no binding (−). Representative gel-filtration profiles for the three scoring classes are illustrated in Fig. S5.
Figure S1. Crystallographic analysis. (a) The asymmetric unit harbors three copies of the mNup98APDyNup82NTDyNup159T heterotrimer that interact with each other in a head-to-tail fashion and are related by an approximate 3-fold axis. A 90°-rotated view is shown on the right. (b) Representative final 2|fo|-|fc| electron density maps, rendered at 1.0 σ, illustrating the three interfaces in the mNup98APDyNup82NTDyNup159T heterotrimer.
Figure S2. Cartoon and schematic representations of the three interfaces in the heterotrimer. (a) FGL-loop interaction. (b) K-loop interaction. (c) yNup159T interaction. Residues are colored according to Figure 4. Van der Waals contacts and polar interactions are represented by dashed and solid black lines, respectively.
Figures from the paper:
Coordinates:
Abstract:
The cytoplasmic filament nucleoporins of the nuclear pore complex (NPC) are critically involved in nuclear export and remodeling of mRNA ribonucleoprotein particles and are associated with various human malignancies. Here, we report the crystal structure of the Nup98 C-terminal autoproteolytic domain, frequently missing from leukemogenic forms of the protein, in complex with the N-terminal domain of Nup82 and the C-terminal tail fragment of Nup159. The Nup82 β propeller serves as a noncooperative binding platform for both binding partners. Interaction of Nup98 with Nup82 occurs through a reciprocal exchange of loop structures. Strikingly, the same Nup98 groove promiscuously interacts with Nup82 and Nup96 in a mutually excusive fashion. Simultaneous disruption of both Nup82 interactions in yeast causes severe defects in mRNA export, while the severing of a single interaction is tolerated. Thus, the cytoplasmic filament network of the NPC is robust, consistent with its essential function in nucleocytoplasmic transport.
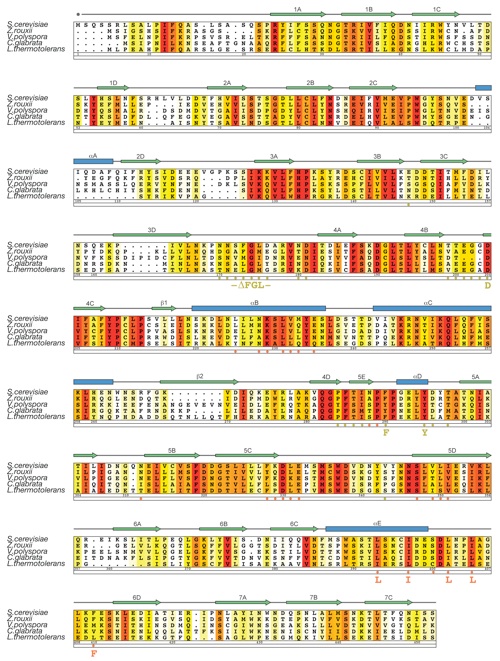
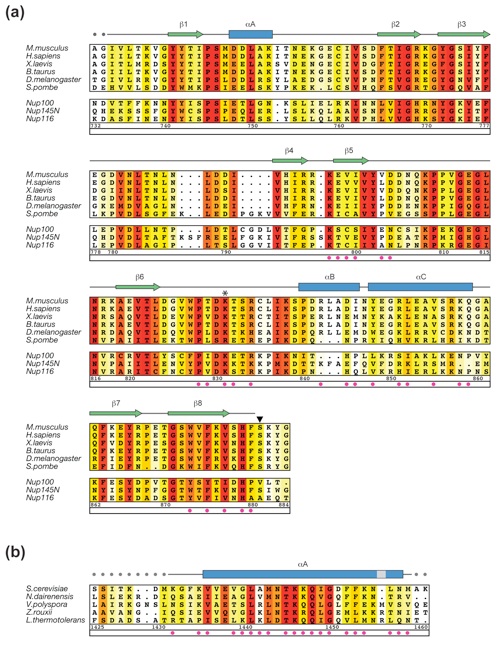
Figure S3. Multi-species sequence alignment for yNup82NTD homologs. The numbering below the alignment is relative to S. cerevisiae Nup82. The overall sequence conservation at each position is shaded in a color gradient from yellow (40 % similarity) to dark red (100 % identity) using the Blosum62 weighting algorithm. The secondary structure is indicated above the sequence as blue boxes (α helices), green arrows (β strands), gray lines (coil regions), and gray dots (disordered residues). Residues involved in the interaction with mNup98APD and yNup159T are indicated by yellow and orange dots, respectively. The positions of the DFY, LILLF, and ΔFGL loop mutations are labeled below the dots.
Figure S5. Quantitation of the size exclusion chromatography interaction analysis. Three representative interactions analysis showing wild-type binding (+), intermediate binding (+/-), and no binding (-).
California Institute of Technology
Division of Chemistry & Chemical Engineering
1200 E. California Blvd.
Pasadena, CA 91125-7200
© Copyright Hoelz Laboratory


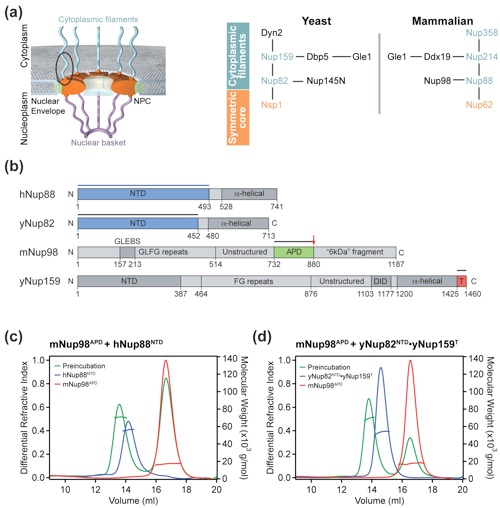
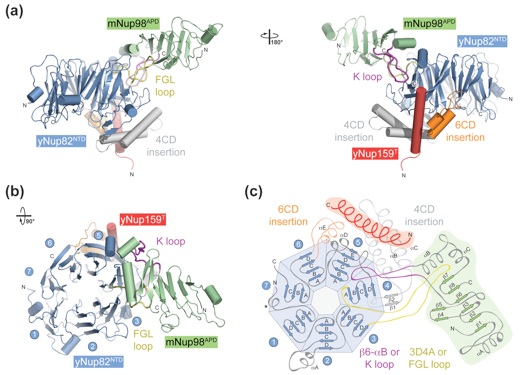
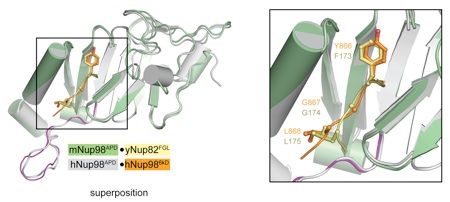
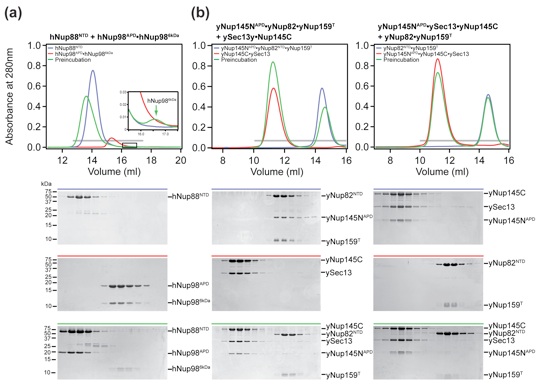
Figure 6. Evolutionary conservation of the binding promiscuity. (a) hNup98APD interacts with hNup986 kDa fragment and hNup88NTD in a mutually exclusive manner. Purified hNup88NTD was mixed at an approximately equimolar ratio with the hNup98APD·hNup986 kDa nucleoporin pair and analyzed by size-exclusion chromatography. The inset shows the displaced hNup986 kDa fragment upon binding of hNup88NTD. Note that the extinction coefficient of hNup986 kDa is very low, resulting in weak absorbance and a small peak. (b) yNup145NAPD interacts with ySec13·yNup145C and yNup82NTD·yNup159T in a mutually exclusive manner. Nucleoporin complexes were mixed at approximate equimolar ratios and analyzed by size-exclusion chromatography. Note that the ySec13·yNup145C pair was used for this experiment, as yNup145C is insoluble in the absence of ySec13. Gray bars and colored lines designate the analyzed fractions. Molecular mass standards and the positions of the proteins are indicated.
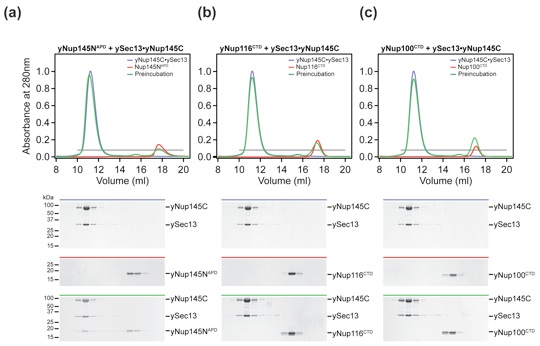
Figure 7. The three GLFG nups of S. cerevisiae are functionally divergent. (a–c) Size-exclusion chromatography interaction analysis of yNup145C·ySec13 with yNup145NAPD (a), with yNup116CTD (b), and with yNup100CTD (c). The analyzed proteins and complexes are indicated in each gel-filtration profile. For analysis of complex formation, the ySec13·yNup145C heterodimer was mixed at approximately 2-fold molar excess of yNup145NAPD, yNup116CTD, and yNup100CTD and injected onto a Superdex 200 10/300 GL gel-filtration column. Gray bars and colored lines designate the analyzed fractions. Molecular mass standards and the positions of the proteins are indicated.
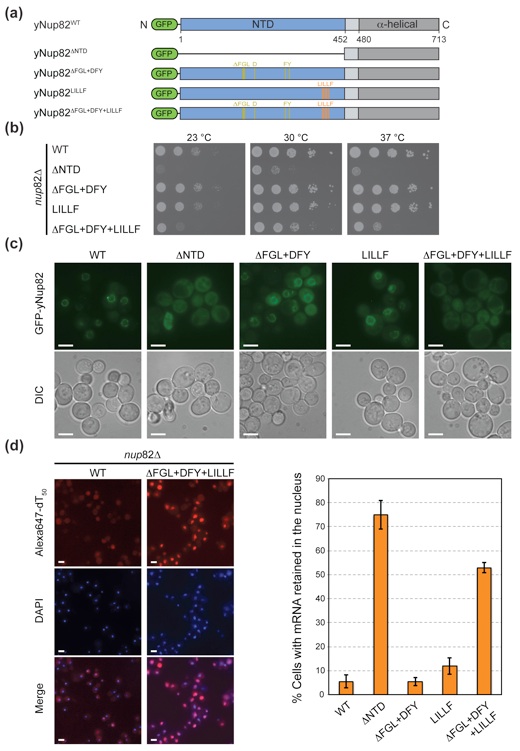
Figure 8. In vivo analysis of Nup82 mutants in S. cerevisiae. (a) Domain structures of the N-terminally GFP-labeled yNup82 constructs. The positions of the ΔFGL, DFY, and LILLF mutations are indicated by lines and colored according to Fig. 5a. (b) Yeast growth analysis using a nup82Δ strain transformed with the indicated GFP-yNup82 constructs. Ten-fold serial dilutions were spotted on SD-Leu plates and grown for 2–3 days at the indicated temperatures. (c) In vivo localization of GFP-yNup82 variants at 37 °C visualized by fluorescence and differential interference contrast microscopy (DIC). (d) mRNA export assay of GFP-yNup82 variants. The detection of poly(A) mRNA and DNA was carried out using an Alexa-647-labeled oligo dT50 FISH probe and 4′,6-diamidino-2-phenylindole (DAPI) stain, respectively. Representative images of wild-type GFP-yNup82 (top) and GFP-yNup82ΔFGL + DFY + LILLF (bottom) complemented nup82Δ cells grown at 37 °C are shown (left panel). Quantitation of nuclear poly(A) mRNA retention is shown on the right. The percentages refer to the fraction of cells displaying a marked nuclear FISH staining. The error bars correspond to the standard deviations that are derived from four independent images. Each image contained approximately 1000 cells. The scale bar represents 5 μm.
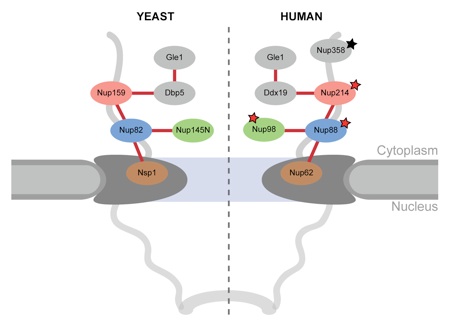
Figure 9. Model for the cytoplasmic filament interaction network of the NPC. Schematic representation of the cytoplasmic filament network of the yeast (left panel) and human (right panel) NPCs. Asterisks indicate nups that are involved in human malignancies (red) and acute necrotizing encephalopathy (black).
Figure S4. Multi-species sequence alignment for mNup98APD and yNup159T homologs. (A) The numbering below the alignment is relative to M. musculus Nup98. The positions of the invariant K loop K831 and catalytic S881 are indicated by an asterisk and an arrowhead, respectively. The three bottom sequences refer to the three GLFG nucleoporins in S. cerevisiae, including the Nup98 homolog yNup145N. (B) The numbering below the alignment is relative to S. cerevisiae Nup159. Residues involved in the interaction with yNup82NTD are indicated by magenta dots. The labeling of the secondary structure elements and conservation coloring scheme of the alignment are according to Figure S3.
Molecular Basis for the Anchoring of Proto-Oncoprotein Nup98
to the Cytoplasmic Face of the Nuclear Pore Complex
Stuwe, T., Schada von Borzyskowski, L., Davenport, A., Hoelz, A.*
(2012). J. Mol. Biol. 419, 330-346.