Hoelz Lab: Publications
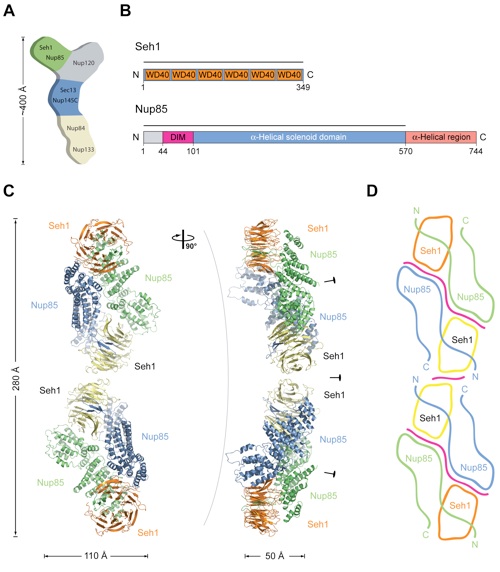
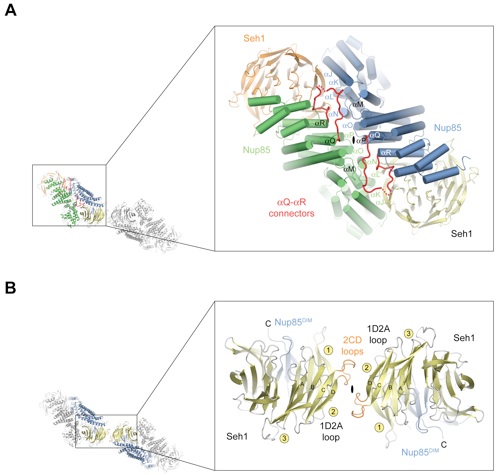
Figure 1. Overview of the Seh1•Nup85 Structure. (A) Schematic representation of the heptameric complex and the approximate localization of its seven nups (Lutzmann et al., 2002). (B) Domain structures of Seh1 and Nup85. For Seh1, the WD40 repeats (orange) and the numbering relative to yeast Seh1 are indicated. For Nup85, the unstructured N-terminal region (gray), the domain invasion motif (DIM) (purple), the -helical solenoid domain (blue), and the C-terminal -helical region (light pink) are indicated. The numbering is relative to yeast Nup85. The bars above the domain structures mark the crystallized fragments. (C) Ribbon representation of the Seh1Nup85 hetero-octamer, showing Seh1 in yellow and orange and Nup85 in green and blue. A 90 rotated view is shown on the right. The three pseudo-twofold axes (black ovals) and the overall dimensions are indicated. The Seh1•Nup85 hetero-octamer forms a slightly bent rod. (D) Schematic representation of the Seh1•Nup85 hetero-octamer. Magenta lines indicate interaction surfaces.

Debler, E.W., Ma, Y., Seo, H.S., Hsia, K.C., Noriega, T.R., Blobel, G., Hoelz, A.
(2008). Mol. Cell 32, 815-826.
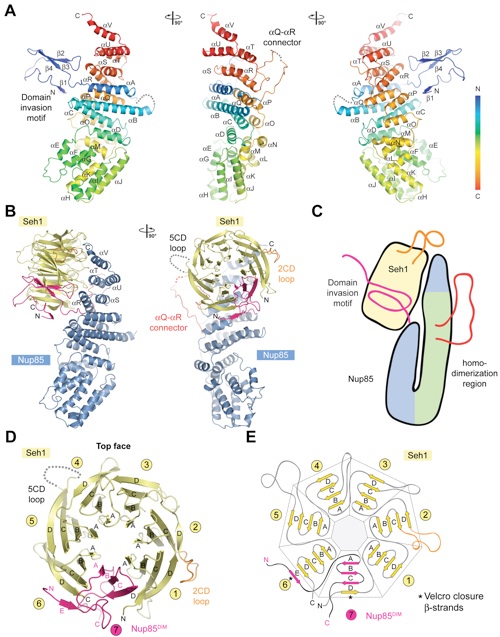
Figure 2. Detailed Structural Analysis of Nup85 and Seh1. (A) A ribbon representation of the Nup85 structure is shown in rainbow colors along the polypeptide chain from the N- to the C-terminus. The N-terminal domain invasion motif (DIM), the -helical solenoid domain, and their secondary structure elements are indicated. (B) The structure of the Seh1•Nup85 heterodimer. The Nup85DIM (magenta), the Nup85 -helical solenoid domain (blue), the Nup85 Q-R connector (red), the Seh1 -propeller (yellow), the disordered Seh1 5CD loop (gray dots), and the Seh1 2CD loop (orange) are indicated; a 90 rotated view is shown on the right. Dotted lines represent disordered regions. (C) Schematic representation of the Seh1•Nup85 interaction. The Seh1 5CD loop, the Nup85 Q-R connector, and DIM region are highlighted in orange, red, and pink, respectively. (D) The -propeller domain of Seh1 in complex with the Nup85DIM. Seh1 is shown in yellow, and the six blades are indicated. The Nup85DIM contributes one strand to blade 6 and three strands to blade 7, completing the -propeller. (E) Schematic representation of the Seh1 -propeller and its interaction with the Nup85DIM.
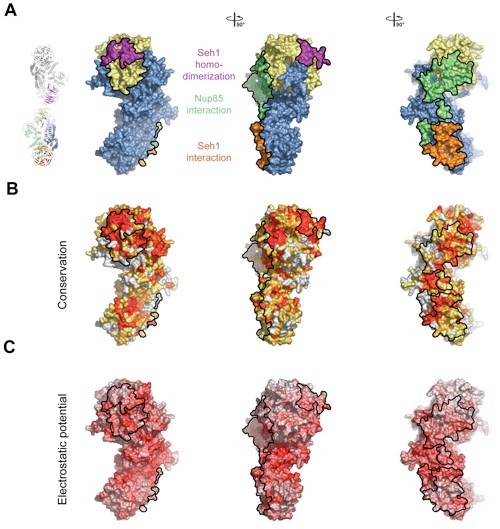
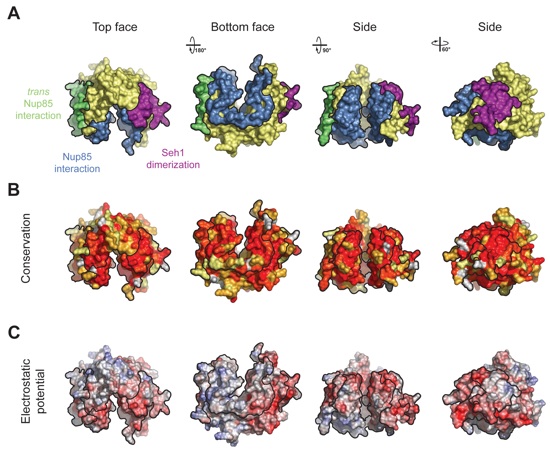
Figure 3. Surface Properties of the Seh1•Nup85 Heterodimer. (A) Surface rendition of the Seh1•Nup85 complex. The surface is colored according to the proteins (Seh1, yellow; Nup85, blue) and their participation in various interactions: with Seh1 of the adjacent heterodimer, orange; with Nup85 of the adjacent heterodimer, green; with Seh1 of the adjacent heterotetramer, purple. (B) Nup85 is colored according to sequence conservation, from 40 % similarity (yellow) to 100 % identity (red). (C) Surface rendition of Nup85, colored according to the electrostatic potential, from red (-15 kBT/e) to blue (+15 kBT/e).
Figure 4. Interfaces in the Seh1•Nup85 Hetero-Octamer. (A) A large surface area of ~5,100 Å2 is buried upon heterotetramer formation and extends over almost the entire length of a Nup85-Seh1 heterodimer. -Helices that mediate heterotetramer formation are indicated. The Nup85 Q-R connector segments (red) are located at the center and contribute many contacts to the interface. (B) The Seh1•Seh1 dimerization interface located at the center of the Seh1•Nup85 hetero-octamer is significantly smaller than the interface in (A). The two interacting Seh1 -propeller domains and the two adjacent Nup85 molecules are colored yellow and blue, respectively. The locations of the pseudo-twofold axes of symmetry that run through both interfaces are indicated (black ovals).
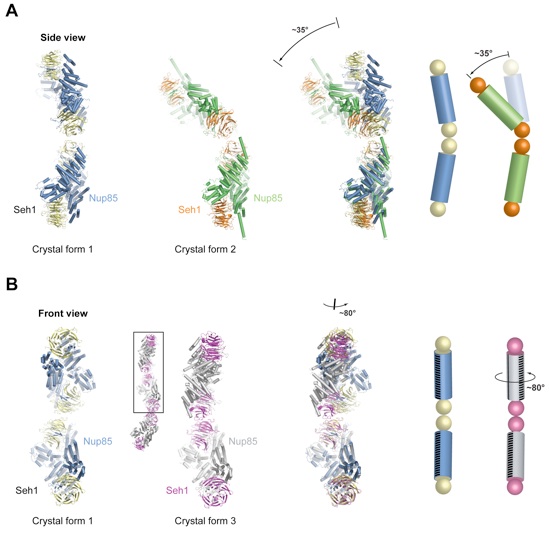
Figure 5. Flexibility of the Seh1•Nup85 Hetero-Octamer. (A) The hetero-octamers of crystal forms 1 and 2 are related by a ~35° hinge motion around the center of the hetero-octamer. On the right, a schematic of the two conformations is shown, where Seh1 and Nup85 are displayed as balls and cylinders, respectively. (B) Crystal form 3 harbors a heterododecamer in the asymmetric unit (small ribbon representation). The two interfaces between the heterotetramers are identical. In the upper two neighboring heterotetramers of crystal form 3 (boxed in the heterododecamer), one heterotetramer is rotated by ~80° around its long axis with respect to crystal form 1. For clarity, a stripe of black lines marks the relative orientations of the heterotetramers. The alignment of the different structures was based on the lower heterotetramer of crystal form 1.
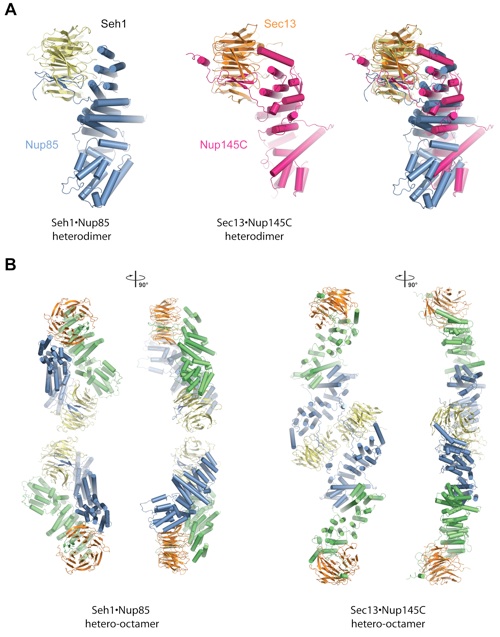
Figure 6. Comparison of Seh1•Nup85 and Sec13•Nup145C. (A) Comparison of the Seh1•Nup85 (left) and the Sec13•Nup145C (middle) heterodimers. Superimposition is based on the -propellers (right). (B) Comparison of the Seh1•Nup85 (first and second panel) and the Sec13•Nup145C hetero-octamers (third and fourth panel).
Figure S1. Experimental Phasing.
PDB coordinates (link to PDB site) - Space Groups P21, P212121, and P21212
PDB coordinates - Space Groups P21 (.pdb), P212121 (.pdb), and P21212 (.pdb)
Structure factors - Space Groups P21 (.txt), P212121 (.txt), P21212 (.txt)
Figures from the paper:
Coordinates:
Abstract:
We recently proposed a cylindrical coat for the nuclear pore membrane in the nuclear pore complex (NPC). This scaffold is generated by multiple copies of seven nucleoporins. Here, we report three crystal structures of the nucleoporin pair Seh1*Nup85, which is part of the coat cylinder. The Seh1*Nup85 assembly bears resemblance in its shape and dimensions to that of another nucleoporin pair, Sec13*Nup145C. Furthermore, the Seh1*Nup85 structures reveal a hinge motion that may facilitate conformational changes in the NPC during import of integral membrane proteins and/or during nucleocytoplasmic transport. We propose that Seh1*Nup85 and Sec13*Nup145C form 16 alternating, vertical rods that are horizontally linked by the three remaining nucleoporins of the coat cylinder. Shared architectural and mechanistic principles with the COPII coat indicate a common evolutionary origin and support the notion that the NPC coat represents another class of membrane coats.
Figure S2. Comparison of the Structures Derived from Three Different Crystal Forms.
Figure S3. Multi-Species Sequence Alignment of Seh1 Homologs.


Figure S4. Multi-Species Sequence Alignment of Nup85 Homologs.
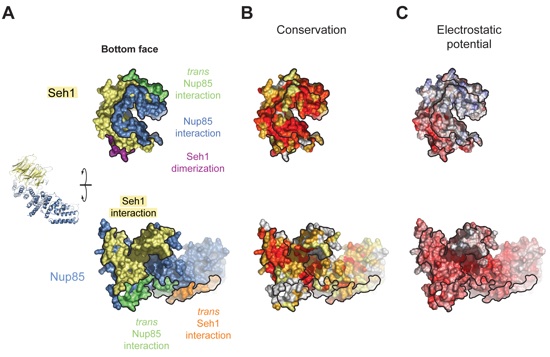
Figure S5. Surface Properties of Seh1.
Figure S6. Surface Properties of the Seh1•Nup85 Interaction.
Figure S8. Biochemical Analysis of the Sec13•Nup85 Complex.
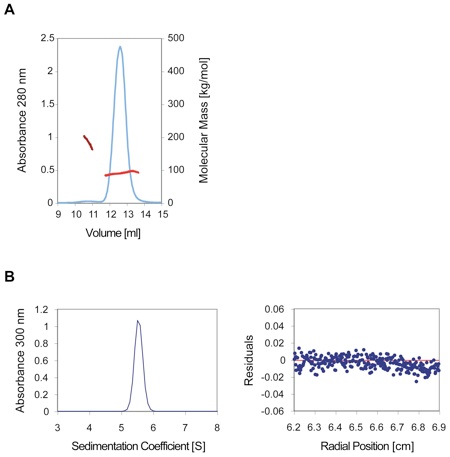
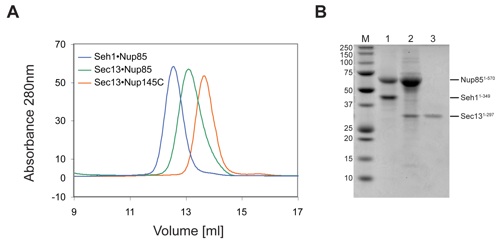
Figure S7. Oligomerization of the Seh1•Nup85 in Solution.

A fence-like coat for the nuclear pore membrane.
California Institute of Technology
Division of Chemistry & Chemical Engineering
1200 E. California Blvd.
Pasadena, CA 91125-7200
© Copyright Hoelz Laboratory
