Hoelz Lab: Publications
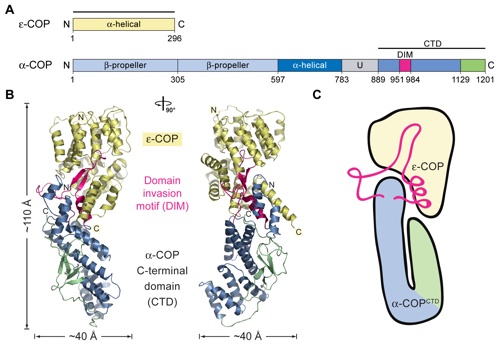
Figure 1. Overview of the structure of the α-COPCTD•ϵ-COP heterodimer. (A) Domain structures of yeast α-COP and ϵ-COP. ϵ-COP is an all-α-helical protein. For α-COP, the two putative N-terminal β-propeller domains (light blue), the α-helical domain (dark blue), the unstructured region (U, gray), and the C-terminal domain (steel blue and green) containing the DIM (magenta) are indicated. The numbering of α-COP and ϵ-COP is relative to the yeast proteins. The bars above the domain structures mark the crystallized fragments and are referred to as the α-COPCTD•ϵ-COP complex. (B) Ribbon representation of the α-COPCTD•ϵ-COP heterodimer colored according to A. The overall dimensions are indicated, and a 90° rotated view is shown on the right. (C) Schematic representation of the α-COPCTD•ϵ-COP heterodimer.

Hsia, K.C., Hoelz, A. (2010). Proc. Natl. Acad. Sci. USA 107, 11271-11276.
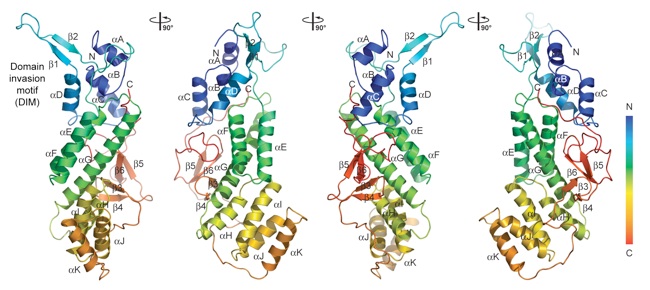
Figure 2. Structure of the α-COP CTD. A ribbon representation of the α-COP CTD structure is shown in rainbow colors along the polypeptide chain from the N to the C terminus. The α-COP CTD has a unique fold with a U-shaped architecture. The α-helical descending arm of the “U”, containing the DIM at the top, the small β-sheet domain that forms the ascending arm of the “U”, as well as their secondary structure elements are indicated. The structure is shown from four different views that are related by 90° rotations.
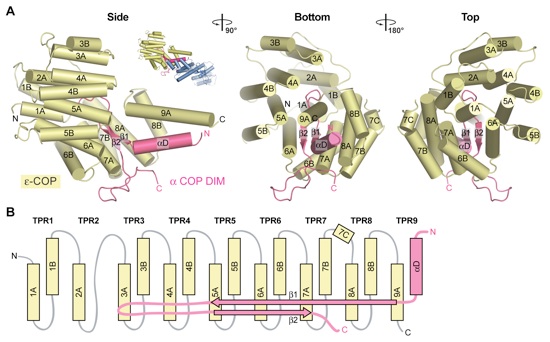
Figure 3. The Interaction of ϵ-COP with the α-COP DIM. (A) ϵ-COP and the α-COP DIM are shown in yellow and magenta, respectively. ϵ-COP adopts a TPR fold that forms an intermolecular TPR with its C-terminal α-helix 9A and helix αD of the α-COP DIM. The β-hairpin of the α-COP DIM is encapsulated by the ϵ-COP TPRs. As a reference, a cartoon representation of the α-COPCTD•ϵ-COP heterodimer is shown and corresponds to the orientation on the left. A 90° rotated view and a view from the top are shown on the right. (B) Schematic representation of the architecture of the ϵ-COP TPRs and their interaction with the α-COP DIM.
Figure 4. The α-COPCTD•ϵ-COP heterodimer binds to a Dsl1 peptide. (A) Gel filtration profiles of the GST-Dsl1410–440 fusion protein (red), the α-COPCTD•ϵ-COP complex (green), and the coinjection of GST-Dsl1410–440 fusion protein with the α-COPCTD•ϵ-COP complex (blue). (B) Gel filtration profiles of the GST-Dsl1410–440 fusion protein (red), ϵ-COP (green), and the elution profile resulting from incubation of the GST-Dsl1410–440 fusion protein with ϵ-COP (blue) prior to injection are indicated. All proteins were injected at approximately the same concentrations.
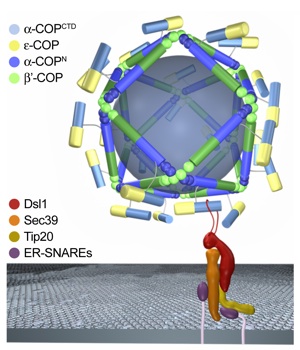
Figure 5. Model for the architecture and ER-targeting of the COPI vesicular coat. Schematic diagram of the outer coat of the COPI complex. The heterotrimeric B-subcomplex of the coatomer that is composed of α-COP, β′-COP, and ϵ-COP is believed to form a coat that is distinct from the hexagonal morphology of clathrin cages. The α-COPCTD•ϵ-COP heterodimer binds to Dsl1, a component of the heterotrimeric Dsl1 tethering complex that targets COPI vesicles to the ER membrane. We propose that the α-COPCTD•ϵ-COP heterodimer is exposed on the COPI coat, while the remaining part of the B-subcomplex, the N-terminal region of α-COP (α-COPN) and β′-COP, oligomerize into a cage with a COPII-like architecture, as discussed in the text. The α-COPCTD•ϵ-COP heterodimer is attached to the cage via an ≈100 residue region that is predicted to be unstructured. For clarity, the heterotetrameric F-subcomplex of the coatomer that shares a similar architecture with the adapter complex and facilitates the oligomerization of clathrin has been omitted. The arrangement of the Dsl1 tethering complex and its interaction with ER-SNARES are according to Ren et al. (36). The interaction of the α-COPCTD•ϵ-COP heterodimer with the exposed Dsl1 loop is shown schematically. Notably, the illustration only reflects one of several possibilities for the placement of α-COPN and β′-COP in a putative COPII-like cage, and, thus, is not meant to reflect the precise position of these proteins.
Figure S1. Sedimentation velocity analyses of the α-COPCTD•ε-COP complex and ε-COP. The sedimentation coefficient distribution shows single species for the α-COPCTD •ε-COP complex and ε-COP (left). For the α-COPCTD •ε-COP complex, the determined molecular mass is 69.2 ` 7.2 kDa, corresponding to a monomeric species of the α-COPCTD •ε-COP heterodimer (calculated 71.9 kDa) in solution. For ε-COP, the determined molecular mass is 37.7 ` 3.1 kDa, corresponding to a monomeric species (calculated 35.4 kDa). No higher-order oligomers are detectable for either samples. The residuals for the fitting are shown on the right.
PDB coordinates (link to PDB site) - 3MV2, 3MV3
PDB coordinates - 3MV2 (.pdb), 3MV3 (.pdb)
Structure factors - 3MV2 (.txt), 3MV3 (.txt)
Figures from the paper:
Coordinates:
Abstract:
The heptameric coatomer complex forms the protein shell of membrane-bound vesicles that are involved in transport from the Golgi to the endoplasmatic reticulum and in intraGolgi trafficking. The heptamer can be dissected into a heterotetrameric F-subcomplex, which displays similarities to the adapter complex of the "inner" coat in clathrin-coated vesicles, and a heterotrimeric B-subcomplex, which is believed to form an "outer" coat with a morphology distinct from that of clathrin-coated vesicles. We have determined the crystal structure of the complex between the C-terminal domain (CTD) of alpha-COP and full-length epsilon-COP, two components of the B-subcomplex, at a 2.9 A resolution. The alpha-COP(CTD) x epsilon-COP heterodimer forms a rod-shaped structure, in which epsilon-COP adopts a tetratricopeptide repeat (TPR) fold that deviates substantially from the canonical superhelical conformation. The alpha-COP CTD adopts a U-shaped architecture that complements the TPR fold of epsilon-COP. The epsilon-COP TPRs form a circular bracelet that wraps around a protruding beta-hairpin of the alpha-COP CTD, thus interlocking the two proteins. The alpha-COP(CTD) x epsilon-COP complex forms heterodimers in solution, and we demonstrate biochemically that the heterodimer directly interacts with the Dsl1 tethering complex. These data suggest that the heterodimer is exposed on COPI vesicles, while the remaining part of the B-subcomplex oligomerizes underneath into a cage.
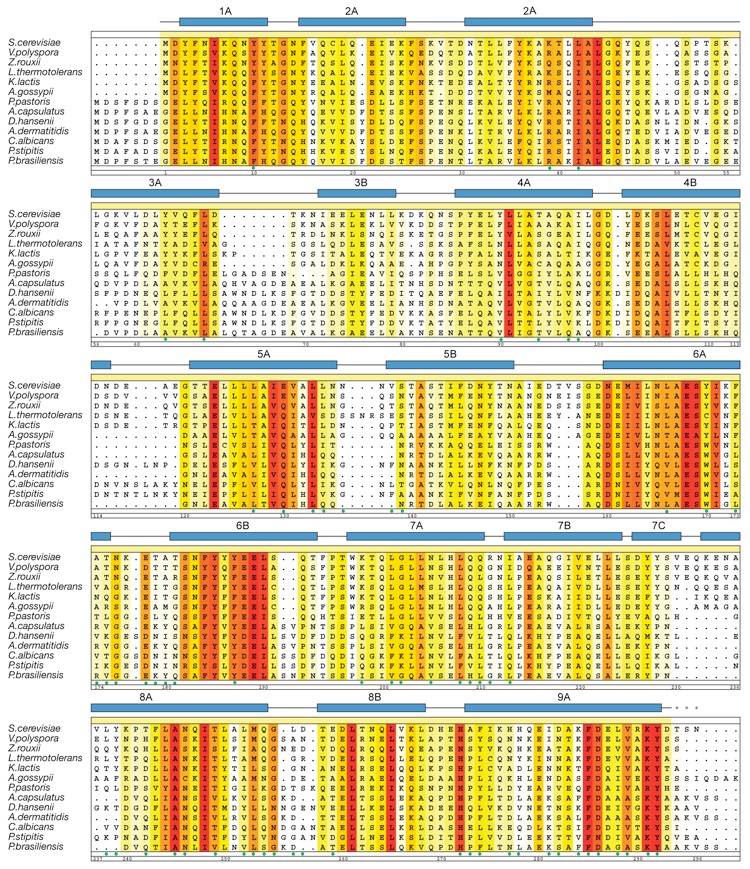
Figure S2. Multispecies sequence alignment of α-COP C-terminal domain (CTD) homologs. The numbering of the residues and the secondary structure are according to yeast α-COP CTD. The secondary structure is indicated above the sequence as green arrows (β-strands), blue rectangles (α-helices), and gray lines (coil regions). The overall sequence conservation at each position is shaded in a color gradient from yellow (60% similarity) to red (100% identity) using the Blosum62 weighting algorithm (1). The α-COP CTD residues that are involved in the interaction with ε-COP are indicated with green dots below the alignment.
Figure S3. Multispecies sequence alignment of ε-COP homologs. The numbering of the residues and the secondary structure are according to yeast ε-COP. The secondary structure is indicated above the sequence as blue rectangles (α-helices), gray lines (coil regions), and gray dots (disordered residues). The overall sequence conservation at each position is shaded in a color gradient from yellow (60% similarity) to red (100% identity) using the Blosum62 weighting algorithm (1). The ε-COP residues that are involved in the interaction with the α-COP CTD are indicated with green dots below the alignment.
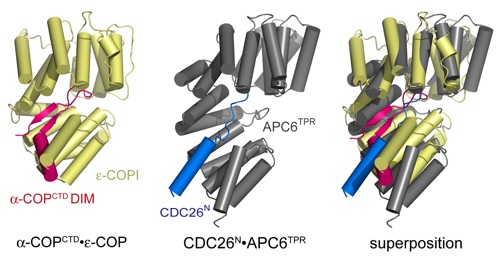
Figure S4. Structural comparison between the α-COPDIM •ε-COP and CDC26N•APC6TPR complexes. The α-COPDIM •ε-COP (left) andthe CDC26N•APC6TPR (middle) are illustrated in magenta and yellow and blue and gray, respectively. A superposition based on the TPRs of ε-COP and the APC6TPR domain is shown on the right. The ε-COP TPRs substantially deviate from the canonical superhelical conformation observed in other TPR proteins (1).
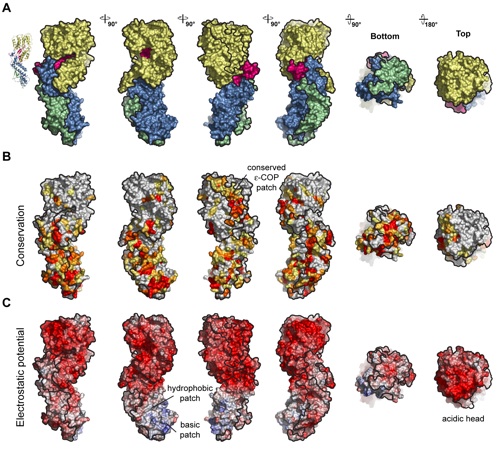
Figure S5. Surface properties of α-COPCTD •ε-COP. (A) Surface renditions of the α-COPCTD •ε-COP heterodimer colored according to Fig. 1B. As a reference, a ribbon representation of the α-COPCTD•ε-COP is shown and corresponds to the orientation on the left. (B) The surface representations of α-COPCTD•ε-COP are colored according to multispecies sequence alignments (Figs. S2 and S3). The conservation at each position is mapped onto the surface and shaded in a color gradient from yellow (60% similarity) to red (100% identity). (C) α-COPCTD • ε-COP surface renditions colored according to the electrostatic potential, ranging from red (−10kBT∕e) to blue (þ10kBT∕e).
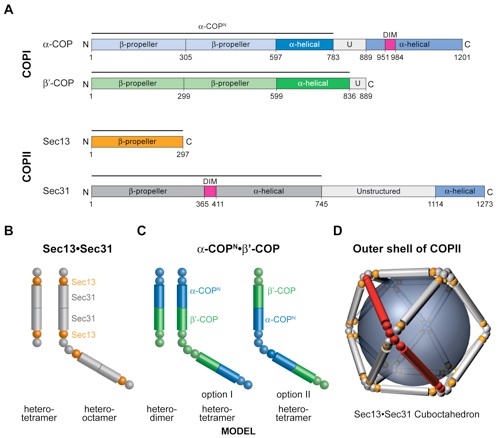
Figure S6. Shared domain architecture of the outer layer COPI and COPII components. (A) Domain structures of yeast α-COP, β′-COP, Sec13, and Sec31. For α-COP, the two putative N-terminal β-propeller domains (light blue), the central α-helical domain (dark blue), the unstructured region (U, gray), and the C-terminal domain (steel blue) containing the domain invasion motif (DIM) (magenta) are indicated. β′-COP has the same domain structure as α-COP but lacks the C-terminal domain. The two putative N-terminal β-propeller domains and the central α-helical domain are colored in light and dark green, respectively. For Sec13, the six-bladed β-propeller is shown (orange). For Sec31, the N-terminal, seven-bladed, β-propeller domain (gray), the Sec13-binding DIM (magenta), the central α-helical domain (gray), the unstructured region (light gray), and the C-terminal domain that is dispensable for coat formation (steel blue) are indicated. The black lines above the domain structures indicate the parts of the proteins that form the structures in B and C. (B) Schematic representation of the Sec13•Sec31 heterotetramer and heterooctamer. The Sec31 DIM facilitates the association with Sec13. The central α-helical domain of Sec31 homodimerizes and forms a heterotetramer. Two heterotetramers form a heterooctamer, which is the repeating unit of COPII cages (1, 2). (C) Schematic representation of the architecture of a putative α-COPN•β′-COP heterodimer and heterotetramer. The heterodimer is formed by the heterodimerization of the central α-helical domains of α-COP and β′-COP. Two heterodimers form a heterotetramer through the homodimerization of the N-terminal β-propeller domain of β′-COP or α-COP. β propellers and α-helical domains are represented by spheres and cylinders, respectively. (D) Sec13•Sec31 heterooctamers oligomerize into the outer coat of COPII with various geometries. Shown is the smallest cuboctahedral cage that is composed of 12 heterooctamers. The central α-helical domains form the edges of the COPII cage. Notably, the COPII vertices are formed by the asymmetric homotetramerization of the N-terminal β-propeller domain of Sec31. For COPI, there are two options for the formation of COPII-like vertices, depending on which N-terminal β-propeller (α-COP or β′-COP) is used in the formation of the heterotetramer. All other arrangements of α-COP and β′-COP would result in cages that are architecturally distinct from COPII.





Crystal structure of α-COP in complex with ε-COP provides insight into the architecture of the COPI vesicular coat.
California Institute of Technology
Division of Chemistry & Chemical Engineering
1200 E. California Blvd.
Pasadena, CA 91125-7200
© Copyright Hoelz Laboratory
