Hoelz Lab: Research
In eukaryotic cells, the spatial segregation of replication and transcription in the nucleus and translation in the cytoplasm imposes the requirement of transporting thousands of macromolecules between these two compartments. Nuclear pore complexes (NPCs) are the sole gateways that facilitate this macromolecular exchange across the nuclear envelope with the help of soluble transport receptors. Whereas the mobile transport machinery is reasonably well understood at the atomic level, a commensurate structural characterization of the NPC has only begun in the past few years.
Our lab carries out structure-function studies of the nuclear pore complex with the ultimate goal of elucidating the atomic structure of the NPC. The applied structure determination as well as the described design principles of the NPC may serve as paradigms for other macromolecular assemblies.


California Institute of Technology
Division of Chemistry & Chemical Engineering
1200 E. California Blvd.
Pasadena, CA 91125-7200
© Copyright Hoelz Laboratory
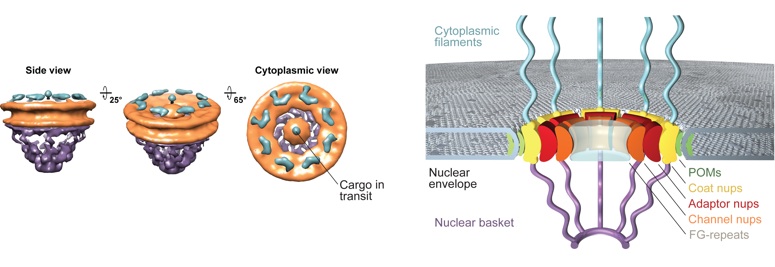
Overall, the nuclear pore complex (NPC) consists of a cylindrical, symmetric core, which is asymmetrically decorated with filaments and a nuclear basket structure on the cytoplasmic and nucleoplasmic sides, respectively. Molecules smaller than ∼40 kDa (small spheres) freely diffuse through the NPC, whereas larger noncargo molecules (large spheres) are prevented from crossing the nuclear envelope.
Inner nuclear membrane (INM) proteins are cotranslationally integrated into the endoplasmic reticulum membrane, which is continuous with the outer nuclear membrane, and then imported to the INM. Similar to the karyopherin-mediated transport in Movie 2, the transport of INM proteins is also dependent on the Ran cycle and karyopherins that likely travel through the central channel, whereas the cargo protein is anchored in the membrane. INM proteins, Kap-α, Kap-β, and RanGTP, are illustrated in light purple, light green, brown, and red, respectively. Substantial structural changes within the NPC would be necessary to facilitate this transport event.
Active import and export of cargoes are facilitated by nuclear localization and nuclear export sequences (NLS and NES, respectively) that are recognized by transport factors, collectively termed karyopherins (Kaps). The NLS of import cargoes (blue) is recognized either directly by an import karyopherin-β (Kap-β; salmon) or via an adapter karyopherin (Kap-α; light green). RanGTP (red) binding inside the nucleus leads to dissociation of the import complex. By contrast, the assembly of a NES-cargo Kap-β export complex requires RanGTP binding (represented in blue, yellow, and red, respectively). In the cytosol, this export complex is dissociated by GTP hydrolysis, which is catalyzed by Ran GTPase-activating protein (RanGAP; dark green) or Ran-binding protein 1 (RanBP1).
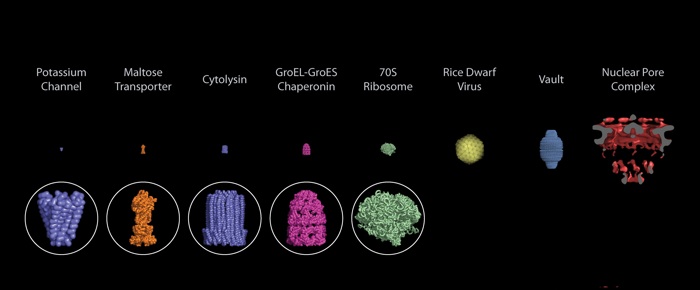
The NPC is nature’s largest and most versatile transport channel. It is an approximately 120MDa complex or about 10 million atoms (30X larger than the ribosome). The NPC is made up of 34 different proteins each occurring in multiple copies totaling 500-1000 individual proteins.